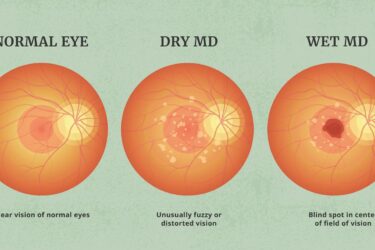
Pegcetacoplan (Syfovre™) for macular degeneration: an update
SBM's guest contributor and ophthalmologist, Dr. David Weinberg, provides an update on the phase 3 trials of pegcetacoplan for macular degeneration. The results are still disappointing.

Pegcetacoplan, a new treatment for macular degeneration
FDA approves a new treatment for macular degeneration: the good, the bad, and the disappointing.
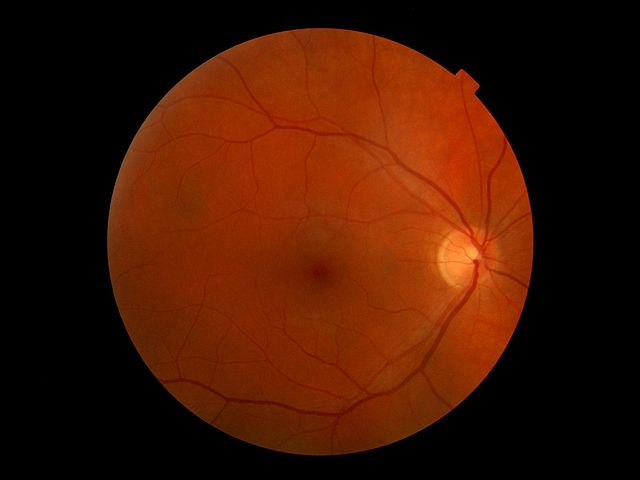
The Stem Cell Ophthalmology Treatment Study (SCOTS): Part 2
Today we continue an analysis of a dubious stem cell clinical trial, with an examination of the clinic and personnel involved.

The Stem Cell Ophthalmology Treatment Study
In this study we have 300 patients with 47 different diseases subject to 7 experimental treatments among 3 treatment groups and no control group. What could possibly go right?
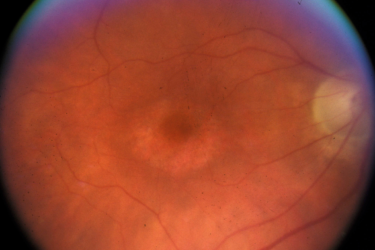
Hydroxychloroquine, retinal toxicity, and COVID-19
Hydroxychloroquine is being promoted as a treatment or prevention for COVID-19 based on questionable evidence. The long-term impact of hydroxychloroquine on the retina introduces another reason to be cautious about its use outside of clinical trials.

The p-hackers toolkit
P-hacking is a common and persistent problem in research, with impacts on the scientific literature, reporting, and practice in medicine. But what is it, really?

Patients Blinded by Stem Cell Therapy: FDA (and consumers) win a legal victory!
The Food and Drug Administration just won a court case supporting the agency's ability to regulate stem cell clinics that rely on client-derived adipose tissues. This is a win for consumer protection, though too late to help those already harmed.
Patients blinded by stem cell therapy: an update
An update on the tragic results of unproven stem cell treatments to treat macular degeneration.
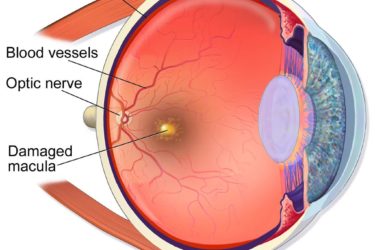
Macular Degeneration, Genes, and Grandma’s Vitamins: To test or not to test?
Is genetic testing necessary to optimize treatment for patients with a potentially blinding eye disease? The stakes are high and the answer depends on which of the two feuding, financially-conflicted groups you believe. In the end, the best evidence wins!

Patients blinded by stem cell therapy: more egregious than you imagined
Three patients were treated with a slurry of stem cells and blood products, in both eyes, on the same day. Could the outcome, three patients becoming legally blind, have been foreseen? Probably.

