
Platelet-Rich Plasma (PRP) Injections: Lots of hype, no convincing evidence
Platelet-rich plasma injections are advertised as an expensive cure-all for sport injuries. The evidence, however, is consistently negative.

Teenager? Anxious? Yes, there’s a supplement for that, too.
Health Canada has criticized the marketing of an "anxiety supplement" for teens, without recognizing the larger problem involved; the poor regulations and lack of safety and efficacy data for this, and many other supplements sold.
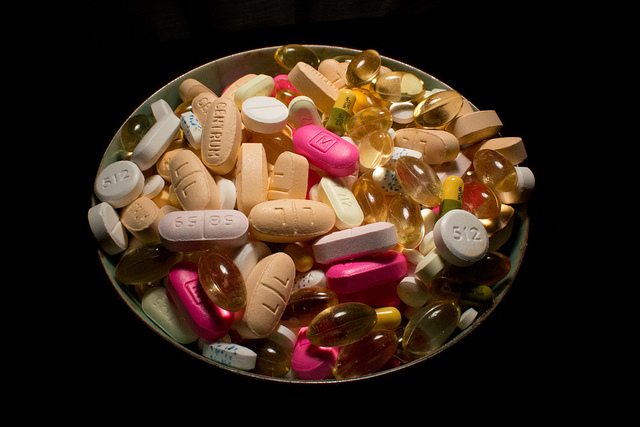
Do dietary supplements improve heart health?
Dietary supplements are widely consumed to improve heart health. But what does the evidence say?

The floor is yours
What topics would you like to see covered at Science-Based Medicine? Open thread today for your suggestions and comments.

Activated charcoal, the wellness scam
Charcoal lemonade is yet another detox scam aimed at separating customers from their money.

CBD Oil: The new miracle cure
Cannabidiol (CBD) oil is hyped as a miracle product to treat virtually everything. What is the evidence to support this?

How effectively does cinnamon treat diabetes?
Cinnamon is often touted as a "natural" supplement that's effective for treating diabetes. The evidence (still) isn't convincing.

Lactation cookies feed on breastfeeding anxieties
There’s little good evidence to say "lactation cookies" do anything at all. If you want cookies, eat cookies. Lactation cookies are an expensive scam.

CAM and cancer: Who uses CAM, and why?
Many patients with cancer use complementary and alternative medicine, and it is important to understand why.

How do you like your coffee? Rectally?
Fill it to the rim? Please don't.

