Category: Clinical Trials

Pegcetacoplan, a new treatment for macular degeneration
FDA approves a new treatment for macular degeneration: the good, the bad, and the disappointing.
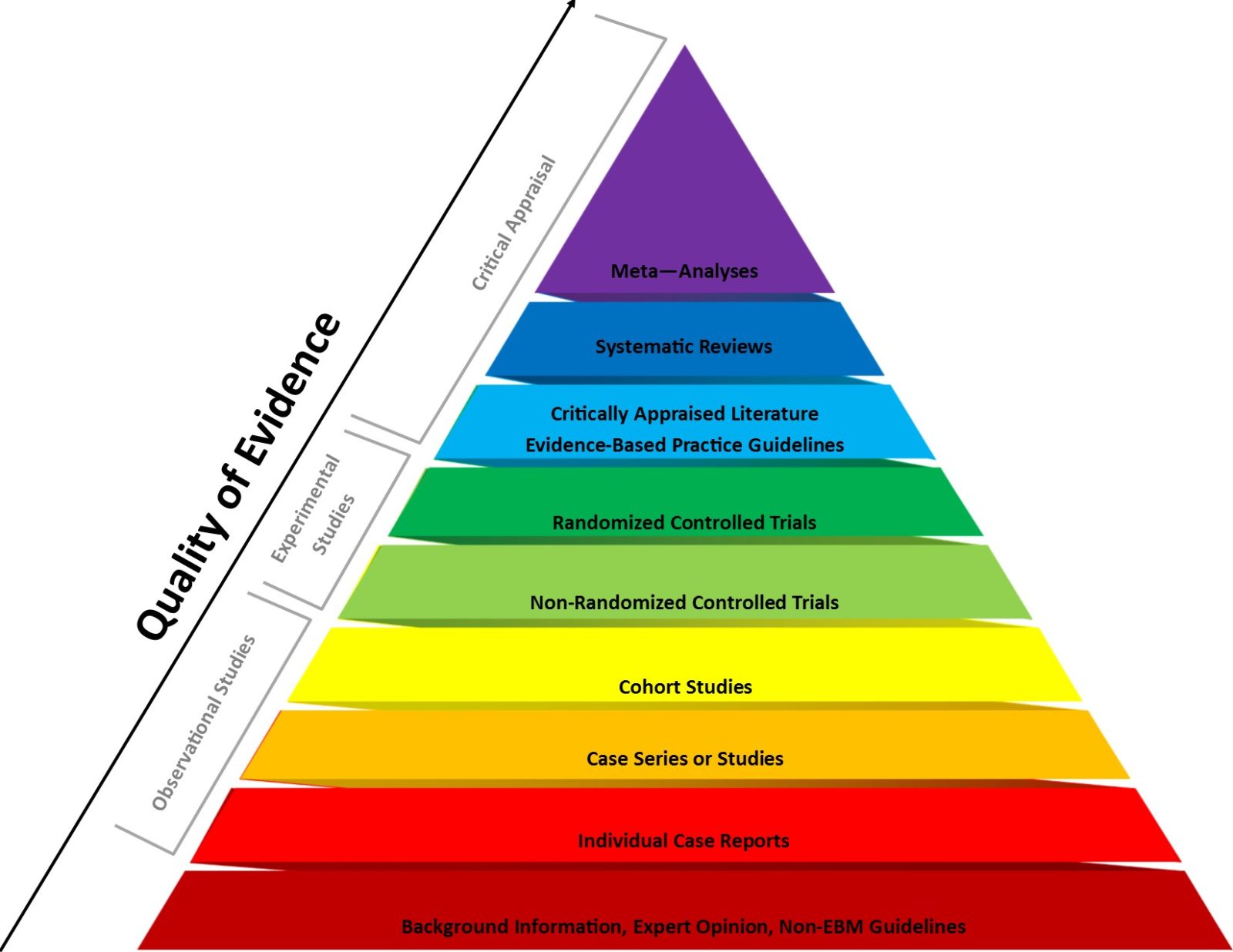
Evidence-based medicine vs. basic science in medical school
Last week Dr. Vinay Prasad wrote a Substack arguing that medical students should learn the principles of evidence-based medicine before basic science.This is a recipe for amplifying the main flaw in EBM that science-based medicine was meant to correct, and Dr. Prasad's arguments would have been right at home on an integrative medicine blog. [Note ADDENDUM.]

Part of a Complete Breakfast
Infection control. When one adheres to compliance it is effective. Like masks and COVID 45.

The Cochrane mask fiasco: How the evidence-based medicine paradigm can produce misleading results
Last week, the Cochrane Collaborative was forced to walk back the conclusions of a review by Tom Jefferson et al that had been spun in the media as proving that "masks don't work." Tom Jefferson himself has been problematic about vaccines for a long time, but the rot goes deeper. What is it about the evidence-based medicine paradigm that results in misleading...
A Global Summit Conclusion: No Evidence of an Effect of SMT [Spinal Manipulative Therapy] for the Management of Non-Musculoskeletal Disorders
A new systematic review places one more nail in the coffin of chiropractic vertebral subluxation theory and the use of spinal manipulation as a treatment for non-musculoskeletal disorders.

Bovine Pancreatic Insufficiency
Repurposed drugs that have multiple mechanisms of action in treating infections usually end up doing nothing. For example, fluvoxamine and COVID 45.

Vaccines and infant mortality rates: A false relationship promoted by the antivaxxers…again, 12 years later
Since COVID-19, in the antivax world everything old is new again. Even hoary chestnuts of bad science used 12 years ago to falsely claim that vaccines kill babies. That's right, Gary S. Goldman and Neil Z. Miller are back to defend their 2011 "study," and RFK Jr. is flogging it as slam-dunk "evidence" that vaccines kill babies.

CBD Oil Fails to Improve Symptom Control in Advanced Cancer
Has the hype outpaced the evidence when it comes to cannabis? A new clinical trial fails to show any benefit of CBD oil in patients with advanced cancer.

Motivation to Exercise in Mice
A new study in mice shows a connection between gut microbiome and willingness to exercise. Could this discovery lead to improved motivation to exercise in humans? Possibly, but we don't yet know if humans share the same pathway.


