Category: Clinical Trials
Corrigendum. The Week in Review for 04/02/2017
Death from vaccine-preventable infections. Homeopathy and acupuncture do not work. There is a difference between cost and worth. And more.

Vitamin C and Sepsis. All Sound and Fury? Much Ado About Nothing?
Is intravenous vitamin C helpful in sepsis? I hope so, but past experience render me skeptical.

Stem Cells for Macular Degeneration: Meticulous Science vs. Unethical Carelessness
Rigorous scientists stabilized a patient’s macular degeneration with a cutting-edge stem cell treatment; less rigorous scientists misapplied stem cell science and left three women blind.

Update on Testosterone Supplementation
Testosterone supplementation is a legitimate treatment for properly-diagnosed androgen deficiency, but it is being overprescribed by doctors who make exaggerated claims for it. New evidence clarifies its modest benefits and worrisome risks.

The Texas Medical Board lets Stanislaw Burzynski off lightly: A cautionary tale of the failure of regulating medicine
After three years and countless twists and turns, the final decision by the Texas Medical Board on the sanctions to be imposed on Houston cancer quack Dr. Stanislaw Burzynski were announced on Friday. Sadly, they were not enough. The Burzynski saga should serve as a cautionary tale that the regulation of physicians and medicine is too lax, not too strict.

Corrigendum. The week in review for 03/05/2017
Canada's Bad Science Wants You. Penguins get acupuncture, tiger cubs get chiropractic. Homeopathic lead for lead toxicity. I'm an idiot. And more
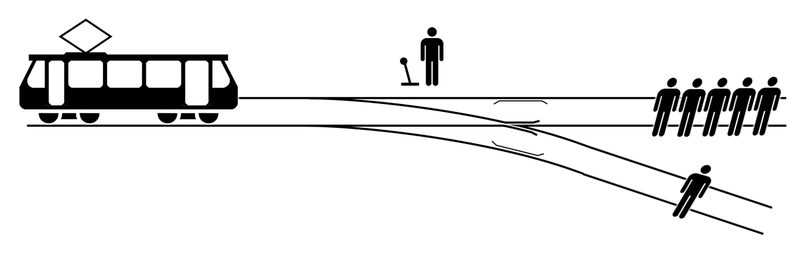
Influenza Vaccine and Health Care Workers. More than one way to skin a literature
There are many ways to apply the medical literature. For me it starts with the premise that health care workers may not injure a human being or, through inaction, allow a human being to come to harm.

Corrigendum. The Week in Review for 02/19/2017
More poorly done acupuncture studies. Burzynski eats just desserts. Italians like homeopathy. New Jersey is going after Oregon. And more
Corrigendum. The week in review for 02/12/2017
The week in review. Chiropractic and stroke. Integrative Medical doctors don’t trust vaccines. Death from medical marijuana. Shilajit: compost or mulch oozing from Himalayan rocks. India goes full Tuskeegee with AIDS. And more!


Spinal Manipulation for Back and Neck Pain: Does It Work? Annotated.
Spinal Manipulation for Back and Neck Pain: Does It Work? You would think it does if you read the article but not if you actually read the literature.