Category: Politics and Regulation

Importing drugs from Canada won’t lower drug prices for Americans
The Trump administration has approved the bulk importation of prescription drugs from Canada. But Canada isn't on board with this plan, and it's not going to reduce drug prices for Americans.

Science-based medical lessons from President Trump’s case of COVID-19 (thus far)
We learned early Friday morning that President Trump has COVID-19. As the story evolved, it was hard not to take a look at potential science-based lessons in medicine that this story provides.

“Health Freedom” group promotes legislation negating masks, other public health measures
National Health Freedom Action is promoting state legislation that would block enforcement of public health measures (e.g., mask wearing and social distancing) during epidemics and other emergencies. The organization and its allies embrace junk science and have successfully passed laws protecting quacks.
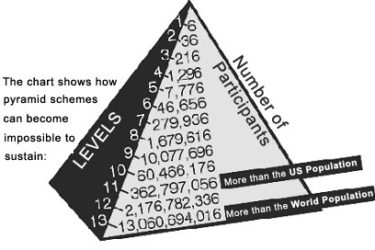
Plexus: MLM Strikes Again
Many health-related products are sold through multilevel marketing (MLM); now the FTC is warning them to stop making false claims about COVID-19. The tactics that MLMs use to promote all their products are deceptive and are a variation on the old Ponzi scam.
In the age of the COVID-19 pandemic, can we trust the CDC and FDA any more?
Since the COVID-19 pandemic reached the US, increasing concern has been expressed about the politicization of the CDC and FDA due to pressure from the Trump administration to downplay the severity of the pandemic and push out treatments and a vaccine as fast as possible, potentially at the expense of safety. This has led me to a disturbing question: Can I trust...

FDA warns companies selling illegal hangover remedies
The FDA recently warned seven companies not to claim that their dietary supplements can prevent, treat, or cure a hangover, because only FDA-approved drugs can make such claims. The agency also warned that NAC, a popular supplement ingredient, cannot legally be used in dietary supplements.
Trump administration announces some COVID-19 tests can skip FDA review, providing new opportunities for dubious lab tests
The Trump administration unexpectedly announced that the FDA will no longer regulate some lab tests, including those for COVID-19. In addition to potentially allowing unreliable COVID tests on the market, the decision creates an opening for more bogus CAM tests.

Does convalescent plasma work against COVID-19? Who knows?
Last night, the FDA issued an emergency use authorization (EUA) for convalescent plasma to treat COVID-19, even though there are no randomized clinical trials demonstrating efficacy and safety. Does this plasma work? Who knows? But that didn't stop the FDA from issuing the EUA, almost certainly as a result of intense political pressure from the Trump Administration.


The Mask Ask: Understanding and Addressing Mask Resistance
The wearing of masks has become contentious on scientific and ideological grounds. Why is that, and how can we communicate with people who don't follow the scientific guidelines?