Category: Politics and Regulation
Misinformation is pervasive, and AI will turbocharge it
Is it possible to refute an infinite amount of AI-generated health misinformation?

Trust in science and vaccines continues to decline. Why?
Recent evidence shows that public trust in science and vaccines has declined markedly since the pandemic. Why is this, and is there anything we can do about it?

Despite safety and quality questions, melatonin use growing in children
A new survey shows use of melatonin in children is widespread despite modest efficacy and an unknown long-term safety profile.

Robert F. Kennedy Jr. comes home to his antivax roots…again
Robert F. Kennedy Jr. gave the keynote speech at the second annual meeting of his antivax organization, Children's Health Defense. Once again, he demonstrated that not only is he still antivax as hell, but that his proposals are even more bizarre than before. Truly, it was a homecoming for him.
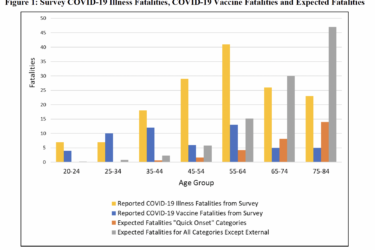
Has MSU economics professor Mark Skidmore been “exonerated” over his retracted paper claiming that COVID vaccines killed 278,000?
Tech bro turned antivax influencer Steve Kirsch is claiming that Michigan State University economist Mark Skidmore has been "exonerated" after having had a paper retracted claiming 278K deaths from COVID-19 vaccines in 2021 alone. In reality, Skidmore just republished a revised version of his retracted paper in an antivax journal after the MSU IRB failed miserably in its oversight duties.

Decongestant doesn’t work, concludes FDA advisory committee
An FDA advisory committee has concluded that phenylephrine, a popular decongestant in cough and cold remedies, is ineffective.

Health misinformation now has powerful allies
Misinformation and conspiracy theories about health had long been a growing problem before the pandemic, but it took COVID-19 to get the government and researchers to take it seriously. Now, a new report in The Washington Post adds to previous reporting from multiple sources describing how allies of misinformation—and not just health misinformation—are striking back under the guise of defending "free speech."

The World Health Organization promotes quackery yet again
The World Health Organization held the First WHO Traditional Medicine Global Summit this weekend. Unfortunately, its claims of being "evidence-based" aside, the conference followed the WHO's usual pattern of serving as propaganda, not science. The summit was one-sided, organized by believers with the only speakers being believers, to promote a predetermined policy goal of promoting traditional medicine and justify "integrating" it with...

The Ohio State Medical Board has finally suspended the medical license of antivax quack Sherri Tenpenny
Last week, the Ohio State Medical Board suspended the medical license of Dr. Sherri Tenpenny, a longtime antivax quack. The only question is: What took them so long, and why did it take the pandemic for them to act? Also, is there less to this action than meets the eye?


