One of the more depressing things about getting much more interested in the debate over how we should screen for common cancers, particularly breast and prostate cancer, is my increasing realization of just how little physicians themselves understand about the complexities involved in weighing the value of such tests. It’s become increasingly apparent to me that most physicians believe that early detection is always good and that it always saves lives, having little or no conception of lead time or length bias. Sadly, just last week, I saw another example of just this phenomenon in the form of an article written by Dr. George Lombardi entitled My Patient, Killed By The New York Times. The depth of Dr. Lombardi’s misunderstanding of screening tests permeates the entire article, which begins with his recounting a story about a patient of his, whose death he blames on The New York Times. After describing the funeral of this 73-year-old man who died of prostate cancer, Dr. Lombardi then makes an accusation:
This one filled me with a special discomfort as I knew a secret: He didn’t have to die. I knew it and he had known it. Had he told?
About 5 years ago he had just retired and had a lot more time on his hands. He was a careful man, lived alone, considered himself well informed. He got into the habit of clipping articles on medical issues and either mailing them to me or bringing them in. They came from a variety of sources and were on a variety of topics. He wasn’t trying to show me up. He was genuinely curious. I kidded him that maybe he’d like to go to medical school in his retirement. ‘No’ he laughed, ‘I just like to be in the know.’
When he came in for his physical in 2008 he told me he’d agree to the DRE but not the PSA (his medical sophistication extended to the use of acronyms: DRE stands for digital rectal exam where I feel the prostate with my gloved finger for any abnormality and PSA for prostatic [sic] specific antigen which is a blood protein unique to the prostate and often elevated in prostate cancer). He had read that the use of PSA as a screening test was controversial. This was the year that the United States Preventive Services Task Force, a government panel that issues screening guidelines, recommended against routine PSA screens for older men. It was often a false positive (the PSA was elevated but there was no cancer), led to unnecessary biopsies, and besides most prostate cancers at his age were indolent and didn’t need to be treated. I countered that prostate cancer was the second leading cause of cancer deaths in men and that it was better to know than not to know. This way it would be our decision. The patient with his doctor deciding what was best. But no, he wanted to stick to his guns and since the DRE was normal no PSA blood test was sent.
After describing a conversation with the man’s daughter, who said, “My father was killed by The New York Times,” Dr. Lombardi then goes on to anecdotal evidence and a cherry-picked publication to support his view, quoting an oncologist who says he’s “seeing more men presenting with advanced prostate cancer” and then referring to a single paper in the current Annals of Internal Medicine about PSA screening. Before I look at the article and a recently published paper on screening mammography that made the news, I can’t help but point out that I (mostly) agree with Dr. Lombardi when he says:
Public health doctors, policy experts and journalists tend to look at the population as a whole. It is a better story if it is one story. It makes a better headline. Their statistics are people I sit across from everyday trying to figure out what the future holds. We each have our job to do.
The problem is, of course, that Dr. Lombardi takes that observation and draws the wrong conclusion, namely that his patient died because of lack of screening. He attacks a straw man, sidestepping the true argument, namely that evidence shows that PSA screening probably causes more harm than good for men at average risk of prostate cancer. Unfortunately, Dr. Lombardi obviously does not understand some very basic concepts behind cancer screening, nor does he apparently recognize that doctors who deal with the population-level data that we have regarding screening tests and try to apply them to individual patients are actually looking in a very systematic way about what the benefits of screening are to the individual patient. More on that later. In the meantime, although I wouldn’t go quite as far as Dr. John Schumann did in criticizing Dr. Lombardi, I do view his lament as a jumping off point to look at some recent data on screening for the two most common cancers, breast and prostate.
The problem with screening (yet again for the umpteenth time)
Before I get to my updates on cancer screening, to set the stage I think it’s critical to revisit these two key concepts that you must understand to understand a bit about the difficulties involved in using a screening test to decrease cancer mortality. I’ve explained them both in depth before, so I don’t feel the need to resurrect a detailed explanation again other than to boil them down to two points, with relevant links and illustrative graphs that illustrate the concept. Those who want the more characteristically (for me) verbose versions can follow the links.
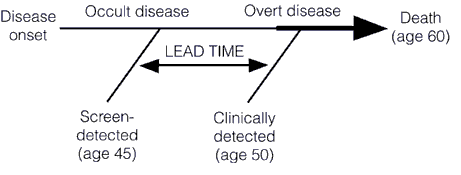
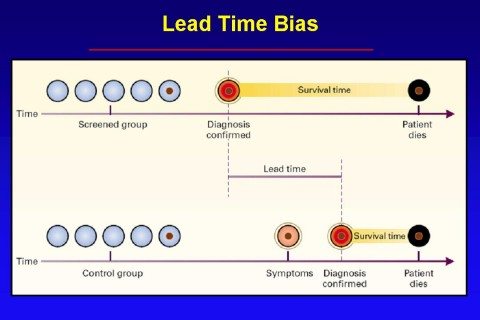
First, let’s briefly recap lead time bias, which tells us that earlier detection does not necessarily result in improvements in survival and more successful treatment. In fact, even if earlier treatment has no effect whatsoever on the natural history of a cancer, earlier detection will still give the appearance of an increase in media survival. That increase is simply the “lead time” that comes from detecting the tumor earlier (hence the term “lead time bias”), before it becomes clinically apparent on its own. This concept is illustrated below in these two graphs, first a simpler one:
And a slightly more complex one:
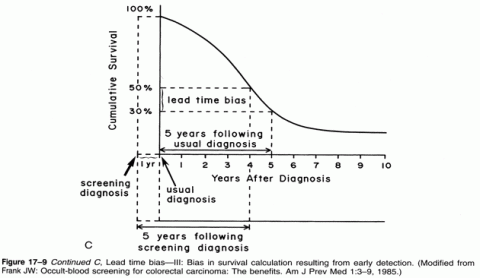
I have explained the concept of lead time bias in more depth here and here, but I do like that graph for illustrating the concept, as well as this one, which demonstrates the effect of lead time bias on a survival curve:
Perhaps my favorite simple and clever explanation of how lead time bias can result in apparently increased survival rates even if treatment has no effect whatsoever on a cancer’s progression comes from Aaron Carroll. It’s well worth reading.
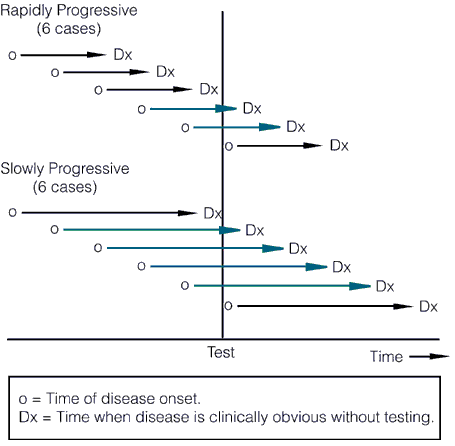
Now let’s briefly move on to length bias. It turns out that most cancer screening tests have an inherent bias towards detecting more indolent, less deadly cancers because of length time bias. Aggressive, fast-growing tumors tend to go from undetectable to clinically apparent due to symptoms, often already having progressed to an advanced stage, in a shorter time than the time interval between screening tests. These tumors thus do not count as having been detected by the screening test because they go from undetectable to symptomatic between, for example, mammography scans. The slower-growing a tumor is, the longer the length of time between its reaching the minimal detectable size of the screening test and becoming symptomatic, the longer the time there is for a screening test to detect it, as illustrated by these two graphs, beginning with this one:
The length of the arrows above represents the length of the detectable preclinical phase, from the time of detectability by the test to clinical detectability. Of six cases of rapidly progressive disease, testing at any single point in time in this hypothetical example would only detect 2/6 tumors, whereas in the case of the slowly progressive tumors 4/6 would be detected. Worse, the effect of length bias increases as the detection threshold of the test is lowered and disease spectrum is broadened to include the cases that are progressing the most slowly, as shown below:
The other problem with length bias is that the more sensitive the test, the more likely it is to detect indolent tumors that are so slow-growing that within the lifetime of the patient they wouldn’t progress to the point where they would endanger the patient’s life. Some, particularly screen-detected cancers, even spontaneously regress. Such indolent or self-limited cancers do not require treatment, but are frequently diagnosed by screening, a phenomenon known as overdiagnosis. The problem, of course, is that our ability to detect such cancers far surpasses our knowledge of how to predict which ones will regress or remain indolent. So we err on the side of aggressive treatment because we quite reasonably view the consequences of guessing wrong as being so much worse for individual patients than overtreating other patients who don’t require treatment. However, for the average patient, the odds of being helped by a screening test are rather small. For instance, to avert one death from breast cancer with mammographic screening for women between the ages of 50-70, 838 women need to be screened over 6 years for a total of 5,866 screening visits, to detect 18 invasive cancers and 6 instances of DCIS. The additional price of this was estimated to be 90 biopsies and 535 recalls for additional imaging, as well as many cancers treated as if they were life threatening when they are not. For prostate, to prevent one death from cancer, 1,410 men need to be screened over 9 years, for a total of 2,397 screening visits and 48 cancers detected. In other words, screening takes a lot of effort for, on an absolute basis, not as many lives saved as we had hoped. Moreover, in the case of breast cancer, on an absolute scale, the reduction in the risk of dying from cancer is small, as I’ve discussed, and the risks of overtreatment are high.
PSA Screening: Smarter, not harder?
Given that Dr. Lombardi cited it, I thought I’d look at the recent Annals article first. The article comes from a group at the Fred Hutchinson Cancer Center at the University of Washington and is entitled Comparative Effectiveness of Alternative Prostate-Specific Antigen-Based Prostate Cancer Screening Strategies: Model Estimates of Potential Benefits and Harms.
I can’t help but notice, first off, that this is a modeling paper. In other words, the investigators did what the hated U.S. Preventative Services Task Force (USPSTF) did in changing its recommendations for mammographic screening for breast cancer and PSA screening for prostate cancer. They took existing data and ran a bunch of computer simulations in order to model the effects of different screening regimens, noting that they were looking for ways to “screen smarter.” Of course, Dr. Lombardi’s argument is basically a straw man in that no one is saying that PSA screening is necessarily completely useless. What is being said is that there is a significant risk of overdiagnosis, overtreatment, and harm that must be balanced against the relatively small benefit of most of the recommended screening regimens. Back when radical prostatectomy was the preferred treatment for early stage prostate cancer, the risk of harm from overtreatment was significant, up to and including death, but more frequently including complications from surgery, such as incontinence, erectile dysfunction, and other less specific risks of major pelvic surgery, such as injury to the colon, bladder, and other bowel. Later, as radiation therapy supplanted surgery as the preferred treatment for very early stage prostate cancer, the potential complications, such as radiation proctitis. Dr. Schumann described these complications dramatically in his rebuttal to Dr. Lombardi’s article.
In other words, a “one size fits all” approach will not do; we need a more “personalized” approach, if you’ll excuse the term in the wake of its corruption by people like Stanislaw Burzynski. That’s exactly what the investigators on this paper (Gulati et al) tried to do.
What Gulati et al did was to model an large number of screening strategies, thirty-five in all, and then estimate the benefits and harms of each compared to the reference screening strategy of annual screening for patients aged 50 to 74 years with a PSA threshold of 4.0 g/L for biopsy referral. They also tried to validate the models when possible by seeing if it predicts incidence beyond the years of calibration (1975-2000) and to perform sensitivity analyses. Gulati et al summarize their results thusly:
Our results yield several important conclusions. First, we find that aggressive screening strategies, particularly those that lower the PSA threshold for biopsy, do reduce prostate cancer mortality relative to the reference strategy. However, the harms of unnecessary biopsies, diagnoses, and treatments may be unacceptable. Quantifying the magnitude of these harms relative to potential gains in lives saved is critical for determining whether the projected harms are acceptable.
Second, we find substantial improvements in the harm/benefit tradeoff of PSA screening with less frequent testing and more conservative criteria for biopsy referral in older men. These approaches preserve the survival effect and markedly reduce screening harms compared with the reference strategy. In particular, using age-specific PSA thresholds for biopsy referral (strategy 20) reduces false-positive results by a relative 25% and overdiagnoses by 30% while preserving 87% of lives saved under the reference strategy. Alternatively, using longer screening intervals for men with low PSA levels (strategy 22) reduces false-positive results by a relative 50% and overdiagnoses by 27% while preserving 83% of lives saved under the reference strategy. These adaptive, personalized strategies represent prototypes for a smarter approach to screening.
It should also be noted that the decrease in risk of dying of prostate cancer, even under the most optimistic scenarios in the models, was on the orders of a fraction of a percent in absolute terms. Under the models studied by Gulati et al, the risk of death due to prostate cancer without screening was 2.86% but could be reduced to between 2.02% and 2.43%, depending on the model. That’s at most a 0.84% absolute risk reduction, although on a relative scale, a decrease in risk of dying from 2.86% to 2.20% is 29%. The overall conclusion from the modeling study was that it is possible to “screen smarter” if the PSA threshold to refer for biopsy is higher, screening for men with low PSA values is decreased in frequency, and older men, who tend to have elevated PSA anyway, are screened less aggressively. All of these are not unreasonable ideas, and they illustrate the tradeoffs involved in any sort of screening program. They are also far removed from Dr. Lombardi’s straw man characterization of arguments that PSA screening can result in more harm than good as “ignorance is bliss when it comes to PSA screening.”
But what about mammography?
We also screen for breast cancer in women. The preferred test, of course, is mammography, and there is no doubt that regular mammography in women between the ages of 50 and 74 definitely reduces mortality from breast cancer. Of that much, we can be certain, although the risks of overtreatment are not insignificant. Over the last decade or so, the recommendations that screening should begin at age 40, that it should be done annually, and that it should continue for the rest of a woman’s life became the basis of public health policy with respect to breast cancer, as well as breast cancer awareness campaigns by advocacy groups. Then, in 2009, the USPSTF dropped its bombshell, in which it suggested that this screening campaign was too aggressive, resulted in too much overdiagnosis and overtreatment, and should be scaled back. Its recommendation was to begin screening for asymptomatic women at average risk (and this must be emphasized: we’re not referring to women at high risk or women who notice a lump in their breast or other symptoms) at age 50 and to perform it every two years instead of every year. I discussed this when the recommendations caused a stir three years ago, and I stand by what I wrote then.
Interestingly, a recent study from the Breast Cancer Surveillance Consortium published in JAMA Internal Medicine a week ago (Outcomes of Screening Mammography by Frequency, Breast Density, and Postmenopausal Hormone Therapy) seems to support the findings of the USPSTF in 2009. It’s a prospective cohort study examining outcomes of women screened at mammography facilities in community practice that participate in the Breast Cancer Surveillance Consortium (BCSC) mammography registries, and the question to be answered was whether there was a difference in outcomes between women screened on a yearly basis with mammography and women who underwent screening on a biennial basis. Data were collected prospectively on 11,474 women with breast cancer and 922,624 without breast cancer who underwent mammography at BCSC facilities, so this is a large study. The study’s findings are summarized thusly:
Mammography biennially vs annually for women aged 50 to 74 years does not increase risk of tumors with advanced stage or large size regardless of women’s breast density or HT use. Among women aged 40 to 49 years with extremely dense breasts, biennial mammography vs annual is associated with increased risk of advanced-stage cancer (odds ratio [OR], 1.89; 95% CI, 1.06-3.39) and large tumors (OR, 2.39; 95% CI, 1.37-4.18). Cumulative probability of a false-positive mammography result was high among women undergoing annual mammography with extremely dense breasts who were either aged 40 to 49 years (65.5%) or used estrogen plus progestogen (65.8%) and was lower among women aged 50 to 74 years who underwent biennial or triennial mammography with scattered fibroglandular densities (30.7% and 21.9%, respectively) or fatty breasts (17.4% and 12.1%, respectively).
Leading the authors to conclude:
Women aged 50 to 74 years, even those with high breast density or HT use, who undergo biennial screening mammography have similar risk of advanced-stage disease and lower cumulative risk of false-positive results than those who undergo annual mammography. When deciding whether to undergo mammography, women aged 40 to 49 years who have extremely dense breasts should be informed that annual mammography may minimize their risk of advanced-stage disease but the cumulative risk of false-positive results is high.
In other words, in terms of reducing their risk of being diagnosed with an advanced stage tumor or a large sized tumor, it appears not to matter whether women between 50 and 74 undergo mammography every year or every other year. This is not the case when it comes to women between 40 and 49, where less frequent screening is associated with a statistically significant increased risk of being diagnosed with advanced disease. However, one has to balance that with the increased risk of false positive mammography and the absolute risk of dying of breast cancer in this age range, which is only in the range of 0.3%. This is a number that increases with age, but it is still small. For instance, Esserman et al characterized it thusly for a 60 year old woman:
Essentially, mammography reduces the odds of a 60-year-old woman dying of breast cancer in the next decade by 30%. Sounds impressive, until you look at her absolute risk: by getting her annual mammogram, her chances of dying from breast cancer are whittled from 0.9% to 0.6%. Overall, for every 1,000 women in their 60s screened for breast cancer in the next 10 years, mammograms will save the lives of 3 people but 6 others will still die. (The numbers edge up or down in lockstep with a woman’s age.)
As is often the case, this study illustrates the difficulty in balancing the risks and benefits of screening for breast cancer. Remember, the idea behind screening for a cancer — any cancer — is that detecting it early will allow early treatment, better prognosis, and a decrease in the number of people who are diagnosed with later stage cancer, which is more likely to be fatal. As a study that I discussed a few months ago showed, mammography has not reduced the number of breast cancer cases diagnosed at an advanced stage as much as one would expect, meaning that there is significant overdiagnosis. Welch’s study estimated overdiagnosis to range from 22% to 31%. From my reading of the literature, this is only a little bit higher than the rate of overdiagnosis of screen-detected breast cancers that has been estimated, namely around 20%.
The bottom line
Does this mean that we should throw up our hands and stop screening for breast and prostate cancer? Of course not! However, we have to balance the risks versus the benefits in a way that doctors like Dr. Lombardi are seemingly unable to do. Dr. Lombardi clearly views screening as an unalloyed good and believes that his unfortunate patient could have been saved if only he hadn’t listened to the NYT, which described the controversy over PSA screening for prostate cancer, and decided that it wasn’t for him. Never mind those pointy-headed docs in the USPSTF who say that PSA screening for men over 75 can’t be recommended and that balance between the benefits and the drawbacks of prostate cancer screening in men younger than age 75 years cannot be assessed, because the available evidence is insufficient. The American Cancer Society emphasizes that the decision to be screened should involve the man being screened. In any case, the danger of relying on anecdotal information, as Dr. Lombardi apparently does, is that there is no good evidence that PSA screening would have saved his patient’s life. Earlier detection could just as easily have ended up with an apparent increase in survival time that was entirely due to lead time bias. Similarly, those of us who take care of breast cancer patients have to be careful not to be seduced by the idea that mammography is some sort of panacea that will give us power over this dread disease.
The idea that finding cancer earlier almost always saves lives is a seductive one. It gives us, the physicians, the idea that we have power over cancer in contrast to how we frequently feel as though we lack such power. While there is no doubt that screening can decrease mortality from cancer, the devil is in the details. Effect sizes and absolute risk reductions are usually very small, even though they might be fairly impressive as relative values. Screening programs are resource intensive. Most importantly, nothing is free. The benefits of screening do not come without a significant cost in terms of money, overdiagnosis and overtreatment, and psychic anguish. It is entirely appropriate to openly discuss these issues, as it is not possible to have true informed consent on the part of the patient if he or she doesn’t understand the potential risks of screening, so that these risks can be balanced against the not-inconsequential benefits that screening could bring.
I’d like to conclude by putting on my scientist cap. I was once a bit like Dr. Lombardi in that I didn’t think much about screening for cancer and assumed that finding disease early is almost always a good thing. I’ve since come to appreciate that, like many things in medicine, it’s not that simple. Medicine is hard and complicated. Real hard and complicated. It’s very rare that any test or treatment is an unalloyed good or ill. Every test or treatment demands a balance between benefits and risks. Screening is no different. If admitting that to our patients and honestly discussing it with them causes us problems as a physician, then it will just have to cause us problems.
To me, the best solution will come from basic research. The shortcomings of screening, including overdiagnosis, overtreatment, lead time bias, and length bias, are not reasons to give up on screening. They are reasons to learn how to screen smarter. They are also reasons that we desperately need to understand the pathophysiology of the diseases for which we screen. After all, after finding a cancer or ductal carcinoma in situ by mammography, it would be so much better to be able to analyze, for example, its gene expression profile, and to predict whether it’s a cancer that will remain indolent or whether it’s one that’s going to progress rapidly. Prediction based on biology, be it biology as determined by genomic profiling, proteomic profiling, metabolomic profiling, or whatever combination of tests it takes, will allow us to usher in the era of true personalized medicine, in which patients receive treatment as aggressive as their disease’s biology requires or no treatment at all, if their disease’s biology tells us that it’s highly unlikely to end their lives or cause serious disability. I only hope I live to see that day.
In the meantime, we muddle through, balancing benefits and risks as best we can and, hopefully, explaining adequately to patients why we do what we do.