Category: Health Fraud
An incomplete list of COVID-19 quackery
Possibly the only thing spreading faster than COVID-19 is the pseudoscience about COVID-19.

Chiropractors continue to make claims about chiropractic and COVID-19
Chirorpractors continue to make unsubstantiated claims about COVID-19.

Chiropractors falsely claim they can protect patients from coronavirus
Chiropractors are falsely claiming that their spinal "adjustments" can protect people from coronavirus infection, as well as giving other dubious health advice on COVID-19. As they have with other bogus remedies, the media and government authorities should take action.

Supplement vendors make unfounded cancer treatment claims
A new analysis shows widespread marketing of natural health products for the prevention or treatment of cancer.

How do we stop crowdfunding sites like GoFundMe from being used to fund quackery?
GoFundMe and other crowdfunding sites have long been used by desperate patients seeking to fund their use of unproven and downright quacky treatments. How can these sites be changed in order to keep them from being used as a funding supply for unethical quacks?

Scientific Fraud in China
There is a systemic problem with fraud in Chinese medical science. The problem goes all the way to the top.

Clínica 0-19: False hope in Monterrey for DIPG patients (Part 5, A dubious poster is presented)
Clínica 0-19 is a clinic run by Instituto de Oncología Intervencionista (IDOI) Drs. Alberto Swiller and Alberto Garcia in Monterrey Mexico that claims to have a much higher rate of survival for patients with DIPG, a deadly brain cancer, than conventional treatments. Patients come there from all over the world for an unproven concoction of chemotherapy drugs administered directly into arteries feeding...
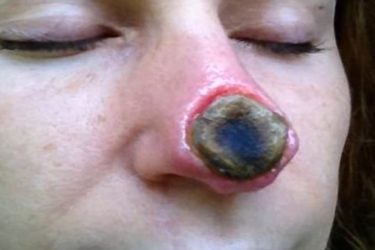
Black Salve Still Thriving Online
Black salve is still dangerous quackery, but it thrives online.

Deconstructing Justice Terry Clackson’s outrageous acquittal of David and Collet Stephans for the death of their son Ezekiel
On September 19, in a retrial ordered by the Supreme Court of Canada, Alberta Justice Terry Clackson issued a ruling acquitting David and Collet Stephan of failing to provide the necessities of life to their son Ezekiel, whose bacterial meningitis they had chosen to treat with quackery instead of medicine, leading to his death in 2012. The news reports showed that this...

Crystal Healing
Crystal healing is back and growing in popularity. What does that reveal about our society and alternative medicine?

