Tag: EBM
2023: The year that the evidence-based medicine (EBM) paradigm was weaponized against vaccines and public health
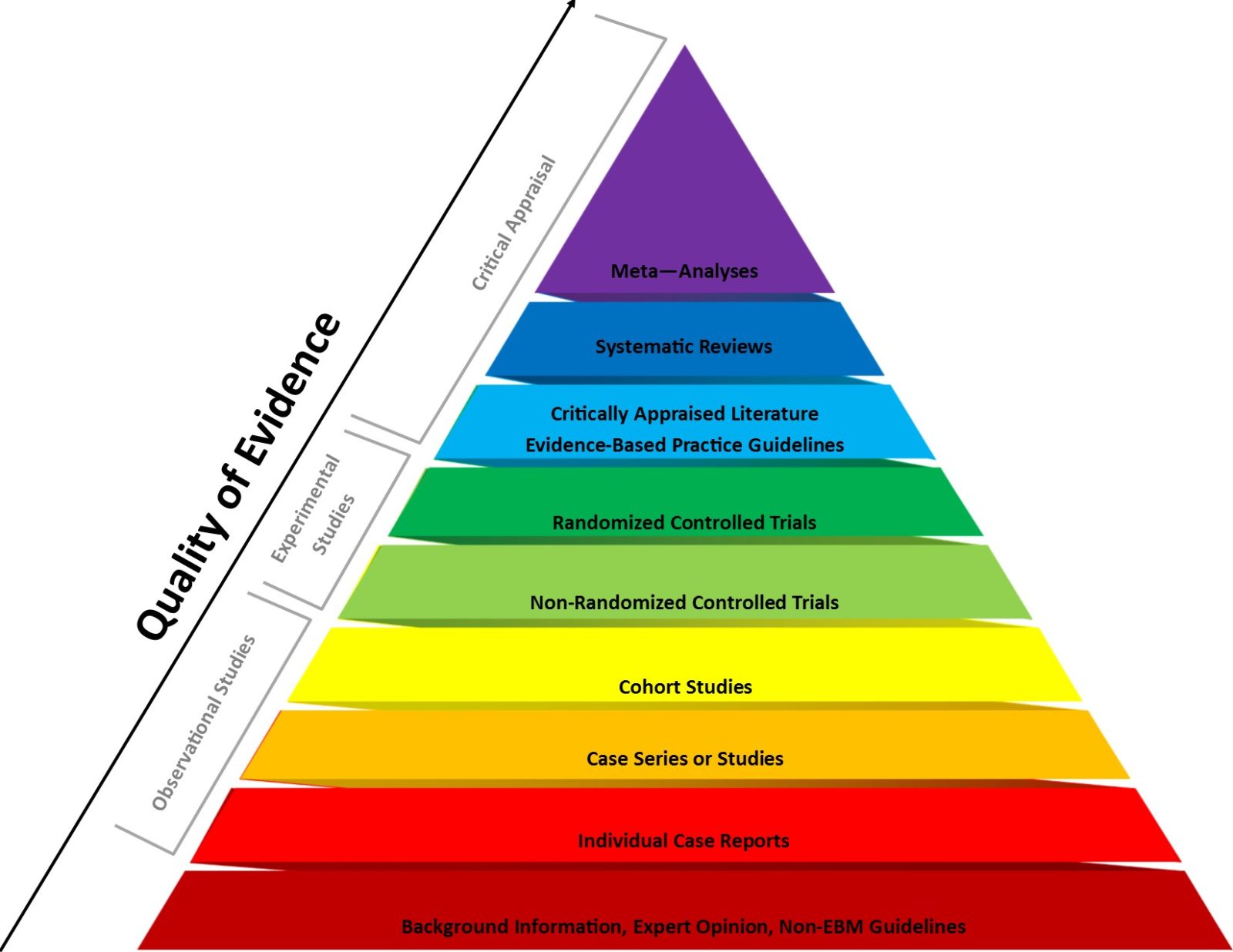
Evidence-based medicine (EBM) has been a very useful paradigm for assessing evidence in medicine. However, like any other framework, it can be misused, particularly when fundamentalist EBM methodolatry leads to its inappropriate application to questions for which it is ill-suited, a misuse that has been weaponized against public health during the pandemic.

