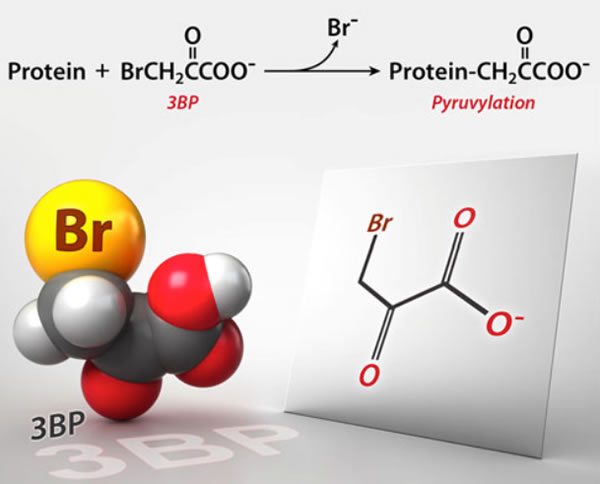
3-BP: A “safe” and “nontoxic” cancer cure targeting the Warburg effect that quite possibly killed three cancer patients in Germany.
There is, however, another country where alternative medicine clinics, particularly for cancer, are common and thriving, specifically Germany. I first learned of these clinics when the story of Farrah Fawcett’s battle with anal cancer hit the news nine years ago. Ultimately, she died of her disease at age 62, but before she did she sought treatment at a clinic in Germany, which administered alternative treatments as well as radioactive seed implants, the latter of which, despite sounding nice and “conventional,” were not standard-of-care for recurrent anal cancer. What this led me to learn is that German alternative cancer clinics tend to use both alternative medicine and experimental “conventional” medicine that has not yet been shown to be safe and effective in clinical trials.
I thought of Farrah Fawcett when news about a German cancer clinic hit the news again beginning more than a week ago, when two patients from the Netherlands and one from Belgium died shortly after having undergone treatment at the Biological Cancer Centre, run by alternative practitioner Klaus Ross in the town of Brüggen, Germany. Two others were hospitalized with life-threatening conditions. I didn’t blog about them at the time because the only reports I could find were those sent to me by readers, and they were in German or Dutch. They also didn’t have a lot of detail. Both reported that on July 25, a 43-year-old Dutch woman went to the Biological Cancer Center in Brüggen-Bracht for treatment of breast cancer and that she unexpectedly died on July 30 of unknown causes. The Dutch report stated that the death occurred under mysterious circumstances and that there were two other deaths, that of a Belgian woman the week before, and a Dutch man.
Elsewhere, Irish newspaper TheJournal.ie reports:
Dutch police, who are supporting the inquiry, appealed for information from other patients, as newspapers reported the clinic had been using an experimental transfusion.
Concern was first raised when a 43-year-old Dutch woman with breast cancer complained of headaches and became confused after being treated at the clinic on 25 July.
She later lost the ability to speak, and died on July 30 although the “cause of her death remains unclear,” the German prosecutors said in a statement earlier this week.
Later, it was learned that the identities of the suspected victims were Joke Van der Kolk, age 43; Leentje Callens, age 55; and Peter van Ouwendorp, age 55.
Unfortunately, the early reports were fairly basic, without much detail, and only a couple with any names. Fortunately, now there is an article in Science that reports more. It turns out that the suspected cause of death is an experimental cancer drug known as 3-bromopyruvate (3-BP) that has not yet been approved for use in humans. So what happened?
What we know thus far
The Science article, published on Friday, reports more than I have been able to discover thus far. First, of all, we learn the treatment that is suspected of having caused the deaths of these three patients and the poisoning of at least two more, 3-BP. Second, we learn that this is a cancer drug that was developed at Johns Hopkins University but has not yet been tested in clinical trials. Then we learn:
The drug in question, 3-Bromopyruvate (3BP), has been hailed by some researchers as a potential breakthrough, but so far the only human data about its efficacy and safety are anecdotal. Many scientists say the drug should not be administered to patients except in carefully controlled experimental settings. If the link to the three deaths is confirmed, that could cloud 3BP’s commercial prospects.
German police took action on 4 August after two patients from the Netherlands and one from Belgium died shortly after undergoing treatment at the Biological Cancer Centre, run by alternative practitioner Klaus Ross in the town of Brüggen, Germany, 50 kilometers west of Düsseldorf. Two other patients had to be treated for life-threatening conditions, the prosecutor’s office said in a press release today. Police in Germany, the Netherlands, and Belgium have urged other patients treated at the center to contact local health authorities; at least 26 have done so. Media reports suggest that cancer patients often sought Ross’s help after they ran out of conventional therapy options, or to avoid aggressive chemotherapy. He offered a 10-week “basic therapy” against cancer for €9900 ($11,057).
On his website, Ross touted 3BP as “currently the best compound to treat tumors.” In its press release, the prosecutor said the investigation has “reinforced” suspicions that the three deceased patients actually received the compound, as media interviews with relatives and other sources had previously suggested.
So what is 3-BP?
3-BP, DCA, and the Warburg effect
Basically, 3-BP is a member of a class of drugs that target a unique feature of cancer cell metabolism, its reliance on glycolysis; i.e., anaerobic metabolism or metabolism not requiring oxygen. Glycolysis is the first enzymatic chemical process through which cells convert glucose to chemical energy in the form of a molecule known as ATP, and does not require oxygen. Indeed, evolutionarily speaking, it’s one of the most ancient metabolic pathways, present in anaerobic and aerobic organisms. It also does not generate very many ATP molecules per molecule of glucose, unlike the next steps, the Krebs’ citric acid cycle and oxidative phosphorylation, the latter of which occurs in the mitochondria, which generate lots of ATP but require oxygen. Basically, anaerobic metabolism is a lot less efficient a source of ATP than is aerobic metabolism.
The details of these processes are taught in basic biochemistry and biology courses the world over, but for the purposes of cancer what’s important is that cancer cells frequently utilize almost exclusively anaerobic metabolism through glycolysis and then utilization of lactic acid. This is known as the Warburg effect after Otto Warburg, who first observed it in 1928. Indeed, rapidly growing malignant cells can have rates of glycolysis up to 200 times that of normal cells. This characteristic of many (but not all) cancer cells is the basis for PET scanning, in which glucose labeled with a positron emitting atom (usually fluorine-18, to produce fluorodeoxyglucose, or FDG) is injected into the bloodstream. Where the FDG tracer accumulates indicates a high level of glycolysis, which makes it useful for detecting metabolically active cancer anywhere in the body. These days, PET scans are often combined with CT scans (PET-CT) in order to show more precisely where the areas of increased FDG uptake are located.
I’ve discussed the Warburg effect before here on SBM in the context of another drug designed to target glycolysis, dichloroacetate (DCA). What drew my attention to this drug initially was a widely-publicized experiment in rats carried out at the University of Alberta by Evangelos Michelakis that was touted by far too many in the press as a major breakthrough in cancer. (Hint: It’s never appropriate to do that based solely on a single animal experiment, however impressive.) Because DCA was a simple small molecule and had been used as a drug before to treat a metabolic disorder, it spawned a unique self-treatment phenomenon. Basically, a pesticide salesman named Jim Tassano bought up DCA from chemical companies in China and sold it as “Pet DCA” and later claimed to be synthesizing pharmaceutical-grade DCA. Of course, with a wink-wink, nudge-nudge, cancer patients found out about DCA, all to treat their pets, of course. Obviously, that wasn’t the intent, and it soon became obvious that cancer patients were buying DCA to treat themselves, despite the fact that at the time the drug had not been tested in humans against any cancer. Ultimately a small phase I trial was carried out adding DCA to standard of care treatment for radiation and showed, as is so frequently the case in phase I trials after promising animal studies, frustratingly equivocal results. Later, at least one Canadian family practice was selling DCA to desperate cancer patients.
DCA also spawned a whole series of conspiracy theories of the type that should be familiar to any regular reader of this blog. The most common one was along the lines of the cure for cancer “they” don’t want you to know about. Because DCA was out of patent and not a lot of pharmaceutical companies were interested in it, it’s not surprising that that would be the dominant conspiracy theory. Today, a quick search of PubMed shows a lot of in vitro work, a couple more small phase I studies, and a single case report of a complete remission in a patient treated with DCA for non-Hodgkin’s lymphoma. It’s also still being sold as a cancer cure.
What does Klaus Ross claim about 3-BP?
Not surprisingly, Ross’ Facebook page and website have been taken down in the wake of these deaths. There are no Archive.org pages, but fortunately the Google Cache still exists. Also, the website for his Biological Centre in the Netherlands is still up. It’s also, unfortunately, all in Dutch, and clicking on the little British flag icon under “Language” doesn’t pull up an English language version. Fortunately, thanks to Google Translate, I got the gist of Ross’ claims for 3-BP, which Ross characterizes as the “current best preparation for tumor treatment” and “more effective than the current chemotherapeutics.” How he knows this, given that there have not been any human clinical trials yet, he does not say, not that that’s stopped quacks before from wildly extrapolating from preclinical studies.
In any case, Ross lays down the usual blather about the Warburg effect, stating that “classical” chemotherapy can harm normal cells, which is of course true, and that it quickly becomes less effective because tumor cells evolve resistance, which is often true depending on the tumor. In contrast, it is implied, 3-BP does not produce resistance. Of course, real cancer researchers know that this is not true. Tumor cells are quick to evolve and adapt, and treatment even with selective metabolic poisons can result in the evolution of resistance. Be that as it may, these are the key claims made by Ross for 3-BP:
- It acts on the energy metabolism of the tumor cell, thus leading to cell death
- It is not toxic to healthy cells and thus does not poison the body
- The immune system is not damaged or weakened
- It has a high selectivity to cancer cells with increased glucose needs
- It does not cause drastic side effects the way chemotherapy or radiotherapy do
Notice how clever Ross is. As you will see, some of these claims are true. For example, 3-BP does act on tumor cell metabolism, does not appear to damage the immune system, and is selective for tumor cells with increased glucose utilization. We don’t know, however, how toxic 3-BP is in humans because the clinical trials haven’t been done yet to show one way or the other the full range of toxicity of the drug. In any event, even if all five of these claims were indisputably true, it would not necessarily mean that 3-BP is an effective anticancer agent. The properties listed are definitely desirable properties in any anticancer drug, but they are not sufficient.
I used to like to point out that several years ago, targeting the Warburg effect was the hot new craze in cancer research. Indeed, attending one of the largest basic science cancer meetings in the world years back, that of the American Association for Cancer Research (AACR), I noticed that everything seemed to be about the Warburg effect and that cancer metabolism was the hot area of research, much as angiogenesis research had been several years before that. And certainly some promising results were reported. Now, several years later, cancer metabolism, while still popular, has taken a back seat to immune checkpoint inhibitor drugs and precision medicine. That’s how cancer research goes. A new discovery is made (or, in the case of the Warburg effect, old knowledge rediscovered in light of new findings), and there is huge enthusiasm that this will be THE breakthrough. After a few years, there’s a reality check, and the limitations of the new discovery become apparent, after which that new discovery is assigned a place in the existing armamentarium of cancer treatments based on data demonstrating its usefulness and limitations.
Of course, one reason why quacks love the Warburg effect so much is that it’s knowledge discovered nearly 80 years ago that was, although not ignored, definitely not given its proper due for decades, only to be “rediscovered” about a decade ago. It also lets alternative medicine practitioners invoke all sorts of woo about “metabolism” and claim that diet somehow targets the Warburg effect, a good example being the so-called “ketogenic diet” touted as “beating chemo for almost all cancers.” (Hint: It doesn’t.) So it doesn’t surprise me at all that a naturopath like Ross would be enamored of 3-BP, particularly since elsewhere he touts cancer treatments involving detoxification, deacidification, correcting the “water balance,” offsetting “mineral deficiency,” supplemental oxygen therapy, craniosacral therapy, high dose vitamin C, MMS, and, of course, “boosting the immune system.”
3-BP: The evidence
As Science points out, 3-BP is a member of a class of small molecule drugs and was first studied as an anticancer agent at Johns Hopkins more than a decade ago by biochemists Young Hee Ko and Peter Pedersen, together with a radiologist named Jeff Geschwind. A couple of early papers showed some promising preclinical results. For instance, this 2001 paper showed that 3-BP induced apoptosis in liver cancer cells. This 2002 paper reported that injection of 3-BP into the appropriate arteries in rabbits could suppress the growth of liver tumors and lung metastases. More impressive, in this 2004 paper, Ko et al treated rats with large hepatocellular carcinoma tumors using 3-BP and reported that in all 19 animals “advanced cancers were eradicated without apparent toxicity or recurrence.” This led one quack cancer clinic to equate this result with that of a human with a “watermelon-sized” tumor on his arm having complete shrinkage and disappearance due to 3-BP. While this comparison is accurate, it is also highly misleading, as it implies that 3-BP can eliminate a watermelon-sized tumor in a human with no toxicity based only on an analogy to a rat study. Don’t believe me? Here’s the graphic:
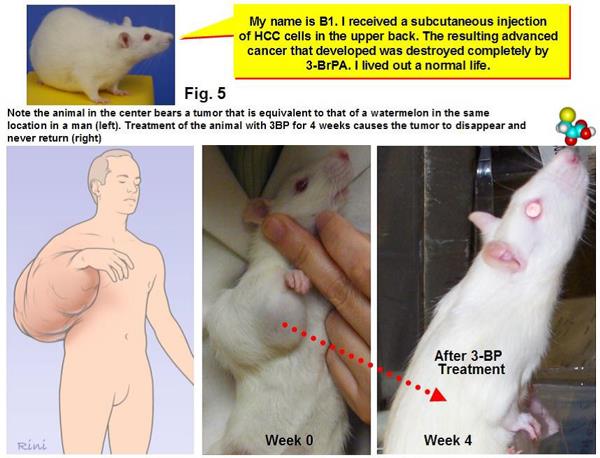
I’ve seen some breathtakingly ignorant and misleading analogies about cancer before, but this one takes the cake. Yes, if you scale up the size of tumor on a rat shrunk by 3-BP, it would be the equivalent of a watermelon-sized tumor on a man. No, because 3-BP eliminate a 3 cm tumor on a rat does not mean that it can eliminate a watermelon-sized tumor on a human.
In 2009, Pederson and Ko teamed up with radiologist Thomas Vogl at the University Hospital Frankfurt in Germany for what appears to be a first experiment in a single patient. With the approval of the local ethics committee, a 16-year-old boy with a rare type of liver cancer called fibrolamellar hepatocellular carcinoma was given infusions of 3BP into the blood vessels supplying the tumor, formulated in a proprietary and patented fashion. Although he died at age 18, the researchers described the experiment as a success in a 2012 paper. “The rate of tumor necrosis due to 3BP treatment seems to exceed all known cytostatic drugs,” they wrote. No major toxic effects were observed, and the patient “was able to survive a much longer period than expected with an improved quality of life, which is clearly attributable to the treatment with 3BP,” the scientists concluded. In retrospect, Vogl says it would have been better to write “probably” instead of “clearly” because a single case report isn’t strong evidence. Still, “we were surprised how long the patient survived and that we saw no toxic side effects,” he says.
Naturally, I looked up Pederson and Ko’s 2012 paper. It describes the aforementioned 16-year-old boy with a rare type of liver cancer called fibrolamellar hepatocellular carcinoma. The authors noted that there is no known treatment for fibrolamellar hepatocellular other than liver transplant and that this patient did not meet the criteria for transplant due to the presence of tumor in the choleductus and spinal lymph nodes. Next, this happened:
Right after the diagnosis, Sorafenib had been approved for primary HCC patients as an orphan drug. Treatment with Sorafenib was started, varying the doses from 200, 400, to 800 mg/day. However, due to lack of experience with such young patients, the treatment outcome was carefully monitored and stopped according to the manufacturer’s instruction when ALT and /or AST values exceeded the tolerable values. Initial results with Sorafenib were encouraging. Thus, tumor growth appeared to have halted during the first 2 months of Sorafenib application, even with some regression of tumor volume. However, after 6 months, CT scans indicated a renewed expansion of the tumor’s mass, and Sorafenib treatment was halted. By now, the health condition of the patient had deteriorated, in fact, so far that he could not consume sufficient amounts of food to sustain life. On the basis of this condition, a duodenal feeding tube was installed and continuous (24 h) enteral feeding was started. The caloric intake was adjusted to 2000–3000 kCalories per day.
So basically the only plausible treatment, Sorafenib, seemed to work for a while but then resistance developed. The next set of treatments was more complicated than described by alternative cancer treatments. It didn’t consist of just intravenous 3-BP but rather other drugs administered by Transcatheter Arterial ChemoEmbolization (TACE), which involves the direct injection into the hepatic artery of various drugs, including cytotoxic chemotherapeutic agents as well as tumor embolization with EmboCept®, which blocks off small arteries feeding the tumor, thus depriving it of blood flow:
Then, the first TACE was performed with Gemcitabine and Cisplatin on January 26, 2009. After delivery of these cytostatics, the blood vessels were blocked temporarily by EmboCept®. Then, the tumor locations were viewed immediately by infusion of Lipiodol® followed by a CT scan. Due to cyto-toxicity, this was the first and last use of these two chemo-drugs. Likewise, the use of Lipiodol® was not continued during 3BP treatment due to a possible interaction between Lipiodol® and 3BP. One month later, permission from the Ethics committee was obtained. The patient was treated immediately via TACE with specially formulated 3BP (patented and proprietary) twice on the first day of treatment (February 26, 2009), a total dose 250 mg. More clearly, the delivery was bolus, meaning a rapid push of the entire dose into the artery over just a few minutes. Therefore, the 3BP delivery can be called a ‘Transcatheter Intra-Arterial Bolus Injection’ followed by a brief emboliza- tion with EmboCept®. The patient did not experience any discomfort during the first treatment with 3BP.
Reading the case report, I would agree with Vogl. Vogl and crew definitely screwed up writing such a definitive conclusion that the patient’s prolonged survival and improved quality of life were “clearly attributable” to 3-BP, and a failure of peer review to let that phrasing stand. That single phrase was the tentpole on which a thousand quacks have been constructing their quack therapies. This should be a lesson to cancer researchers with promising new treatments to be more circumspect in what they write because, not surprisingly, a more recent paper in 2014 described the treatment of a 28 year old man with metastatic melanoma in which the antitumor effect observed was minimal. The drug did, however, have an acceptable toxicity profile.
In brief, what we have here is a drug that shows some promising results in preclinical studies involving cell culture and animal tumors based on a method that targets tumors with high glucose utilization. In science-based medicine (and evidence-based medicine), the next step, given that this treatment has a fairly high degree of biologic plausibility based on mechanism and preclinical studies, would be to do a phase I clinical trial. That hasn’t been done yet, but is planned, according to Science:
But 3BP has yet to undergo formal clinical trials. PreScience Labs, a U.S. company founded by Geschwind, received approval for a phase I study from the U.S. Food and Drug Administration in 2013. Geschwind says the trial has yet to start because the company needs partners to finance it.
So, basically, outside of a clinical trial or a single-patient trial such as the two described above performed with the approval of an ethics committee, 3-BP should not be used in patients with cancer, particularly given that we don’t know its effects in humans when infused intravenously.
None of that has stopped the quacks, though.
Conspiracy theories and a new DCA
3-BP appears to be fast becoming DCA all over again. The “alternative” cancer cure press has been publishing articles with titles like “Cancer Cured, Again“, “Cancer Cure Found by Dr. Ko at JHU“, and, of course, the ever popular “Is a Cancer Cure Being Suppressed?” In each case, as happened for DCA, it’s insinuated (or outright claimed) that 3-BP is a drug that cures cancer but the pharmaceutical companies are “suppressing” it to protect their profits. It’s all a predictable game plan. For example:
A decade has passed since that first animal lab experiment and humanity is still waiting for a human trial to commence in what may be THE CURE for cancer.
The delays seem to be more commercial than scientific. Researchers have scrambled to make patentable analogs (synthetic look-alike molecules) that work better or at least do the same thing. It appears millions of cancer patients may be needlessly dying while researchers battle it out on the commercial front to gain patents.
Virtually the same thing was written on hundreds of quack websites a decade ago, when DCA was the latest darling “suppressed cancer cure.” It doesn’t help that apparently there is some sort of patent issue going on between Ko and JHU. Of course, when a university investigator discovers something while working for a university, the university owns the patent, usually giving the investigator a share of the credit on the patent and a share of the revenue generated if that patent is purchased and leads to a product. I also note that at the time of the discovery of 3-BP, Ko was first author, which strongly suggests to me that she was a graduate student or postdoc and not an independent investigator. Unfortunately, not all principal investigators cut their underlings in when they discover something working in their lab. My PI was nice enough to cut me in on a discovery I made while working as a graduate student in his lab back in the early 1990s, but he was under no obligation to do so. I can’t help but speculate that this might be what’s going on when the above article claims that JHU is trying to develop 3-BP without Ko.
Unfortunately, drugs like DCA and 3-BP, which are easily synthesized and can be obtained from a number of chemical suppliers, are like catnip to quack clinics, which can buy the chemicals cheaply and administer them, particularly German cancer clinics. Such cancer clinics are loosely regulated in Germany, and they specialize in all manner of cancer quackery in comfortable, spa-like facilities. However, they also seem to like to latch on to experimental cancer treatments like 3-BP that have not been validated yet. Whether it’s to appear “cutting edge” or simply because they sense an excellent profit center is unclear. Unfortunately, the damage done by this practice is not just to the patients. As I pointed out with DCA, the widespread use of 3-BP leading to complications such as these deaths of patients under Ross’ care could endanger its clinical trials and approval. After all, what companies or venture capital firms would want to partner to fund studies of 3-BP after this news? If 3-BP is really an effective anticancer treatment in humans, this recklessness on the part of alternative cancer clinics could deny cancer patients a truly effective drug by tainting it with their association.
Klaus Ross might now be banned from practicing in the district where his cancer clinic is located, but there are plenty of other quack clinics offering 3-BP to continue the potential harm, even in the good old USA.
