Category: Clinical Trials
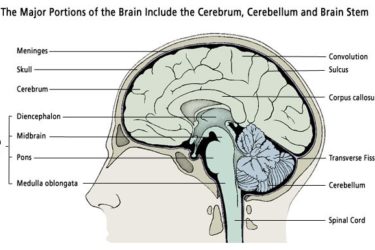
FDA Approves Controversial ALS Drug – Relyvrio
A close look a the FDA approval of Relyvrio.
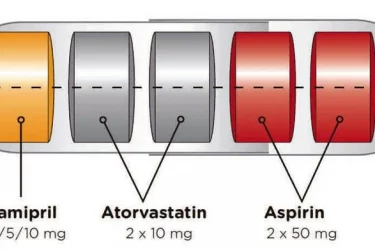
The Polypill Comes of Age
The polypill is effective for secondary prevention of cardiovascular disease. By combining drugs in a single pill, it improves convenience and compliance.
Does Vitamin D Prevent Autoimmune Disease?
A new study published in The BMJ suggests vitamin D might prevent autoimmune disease, but there are reasons to be cautious.
Peer review fail: Vaccine publishes antivax propaganda disguised as “reanalyses” of Pfizer and Moderna COVID-19 vaccine clinical trial data
In order portray COVID-19 vaccines as dangerous, Peter Doshi has now managed to get poorly designed and performed "reanalyses" of the clinical trial data used by the FDA to grant emergency use approval of the Pfizer and Moderna vaccines published in two reputable journals, The BMJ and Vaccine? What happened?

PreP for HIV/AIDS
Two pills and an injection are FDA-approved to prevent HIV infection. Not enough patients and providers know about them.

Is Olive Oil Good Medicine?
A study found that olive oil increased longevity and reduced the risk of cardiovascular disease, cancer, and other chronic diseases, but it only showed correlation, not causation.
Statistical Shenanigans?
The manufacturers of Covid-19 vaccines say they are 95% effective. Peter Doshi re-examined the evidence and estimates they are only 19-29% effective. This pre-print of an as-yet unpublished re-analysis raises many questions but doesn't support the claims being made on antivaccine sites.

Apples, Oranges, and How Not to Analyze a Vaccine RCT
The evidence is overwhelming that COVID vaccines keep people alive and out of the hospital. Only someone who starts with the conclusion that vaccines don't work and then works backwards to find the evidence could claim otherwise.
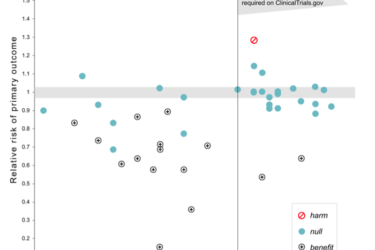
Homeopathy and Pre-Registered Trials
Preregistering clinical trials is a great idea, but we have to actually track registration.