Category: Medical devices
False balance in an NBC news story on whole body MRI scans
Over the weekend, NBC News aired a story on whole body MRI scans. Although it did include the usual cautions about false positives and the harm they cause, the caution was diluted by the story's focus a rare case of a woman who had a brain tumor detected. Overall, it was false balance that reminded me of vaccine/autism stories 20 years ago.
Novak Djokovic credits the TaoPatch for his success: What does the science say?
His ‘Biggest Secret’ is a tiny sticker selling for hundreds of dollars

Can Kinesiology Tape Increase Oxygen Delivery and Improve Sports Performance?
USA Track & Field is endorsing a kinesiology tape that is claimed to improve cell oxygenation.

Earthing Update
Earthing is yet another dubious medical claim exploiting weaknesses in the system.

Treating Nightmares with a Smart Watch
Preliminary evidence suggests a watch may help people with severe nightmares. Of course, more research is needed.

Joovv and Other Red Light Therapies
The Joovv Go is a handheld device for red light therapy. Red light therapy remains controversial: most of the claims are not supported by credible scientific evidence.
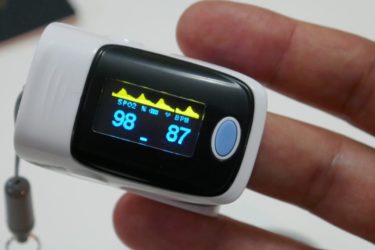
COVID-19 puts the spotlight on an unexpected racial disparity in health care
Evidence increasingly suggests that pulse oximeters, the little finger clips that measure blood oxygen, overestimate the blood oxygenation in Black patients. It's a problem that's been discussed a long time that took a pandemic to bring to public consciousness. How can SBM decrease or eliminate such healthcare disparities?

Signos Sells a Continuous Glucose Monitor, But Not to Diabetics
Signos is asking customers to pay for the privilege of testing their glucose monitoring system.

Plenity – A New Weight Loss Pill
Plenity is a new weight loss pill designed to create a sense of fullness. It is backed by a single study where users had an average weight loss of 22 pounds. Not an effective way to achieve ideal weight, but may help some people when combined with diet and exercise.
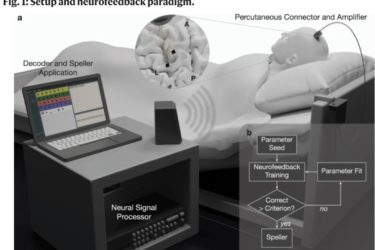
Paralyzed Patient Communicates with Brain Implant
Brain-machine interfaces continue to advance.

