Category: Politics and Regulation

The stem cell hard sell, Stemedica edition
Stemedica Cell Technologies, a San Diego company that markets stem cell treatments for all manner of ailments, likes to represent itself as very much science-based. There are very good reasons to question that characterization, based on the histories of the people who run the company, as well as the company's behavior.

Fixing the supplement market for consumers
When it comes to regulating and selling dietary supplements, should consumer interests be higher priority than those of manufacturers? While regulations are seemingly created to protect consumers, governments around the world have consistently given manufacturers the upper hand, prioritizing a company’s desire to sell a product over a consumer’s right to a marketplace with safe, effective products. Nowhere is this more the...
The Fluoride Dragon Cometh! Or does he?
[Editor’s note: With no further ado, and with no introduction necessary, here is a second post from Craig Pearcey; Witness his science and despair, quacks of the world!] First for the basic chemistry There is one particular word that tends to get many CAM supporters very vocal and the conspiracists thinking about running for their home-made bunkers in a basement somewhere. It...

FDA Looks At Dubious Stem Cell Clinics
Using stem cells to treat disease or improve recovery is an exciting area of research. The potential is undeniably great – these are cells that have the potential to differentiate into mature cells of a specific type. They can be used to replace damaged cells or improve the environment for cell function and recovery. Ideally stem cells can be developed from cells...

Alternative Medicine Is Infiltrating Veterinary Continuing Education
My friend Carmen Czachor is a science-based veterinarian practicing in Port Angeles, Washington. She has alerted me to a disturbing development that she fears will “put veterinary medicine back in the dark ages.” The Washington State Department of Health is contemplating a rule change in the regulations requiring continuing education for veterinarians. Current requirements are for 30 hours of continuing education every...
“Non-pharmacological treatments for pain” ≠ CAM, no matter how much NCCIH wishes it so
When it comes to pain, in the mythos of "complementary and alternative medicine" (CAM), which in recent years has morphed into "integrative medicine," anything that isn't a drug is automatically rebranded as CAM, whether it's in any way "alternative" or not.
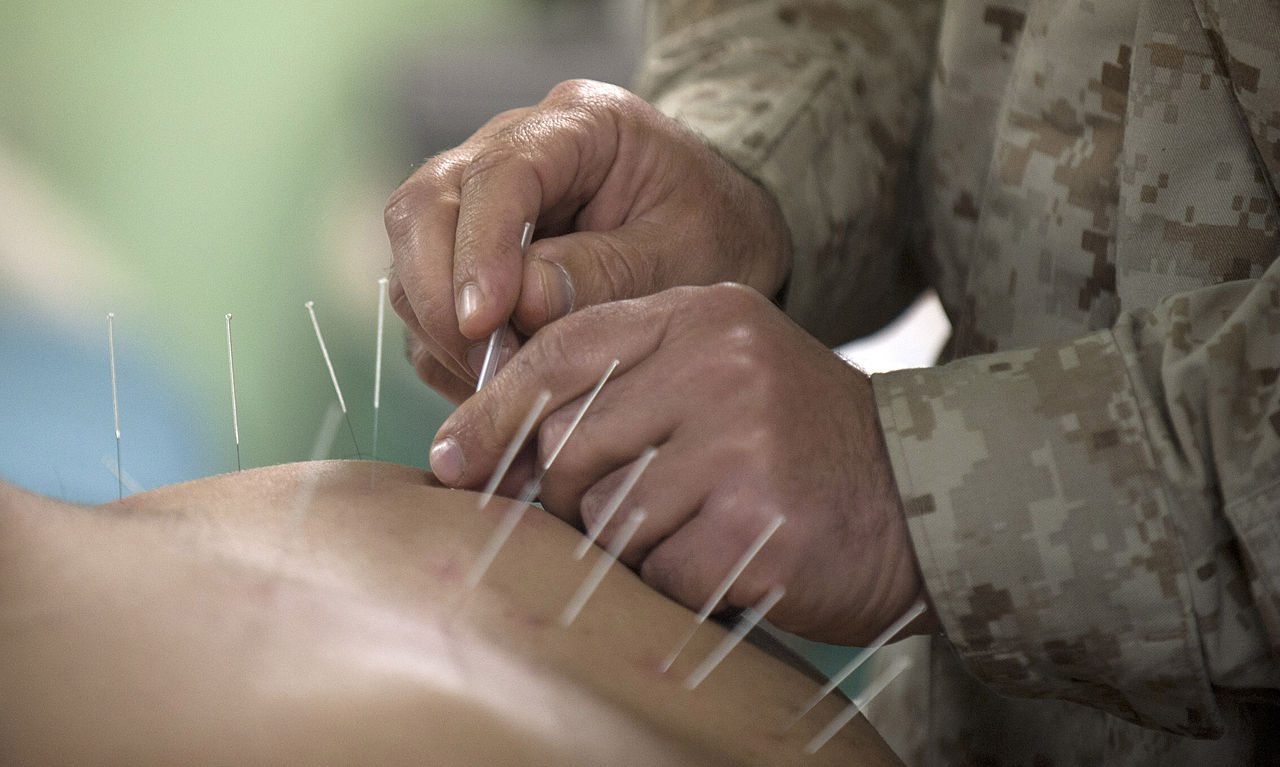
Nada for NADA: “acudetox” not effective in addiction treatment
The National Acupuncture Detoxification Association (NADA) teaches and promotes a standardized auricular acupuncture protocol, sometimes called “acudetox.” NADA claims acudetox encourages community wellness . . . for behavioral health, including addictions, mental health, and disaster & emotional trauma. I do not know what “community wellness” is or how one measures whether wellness has been successfully “encouraged.” In any event, in the NADA...

Good Thinking Society’s successful challenge to NHS homeopathy
[Editor’s note: For no reason whatsoever other than to share great news, we bring you this contribution from Michael Marshall, project director of the Good Thinking Society and vice president of the Merseyside Skeptics Society.] As regular readers of this blog may know, skeptics here in the UK have been campaigning for some time to end the funding of homeopathic remedies by...



