Category: Politics and Regulation

The State of Florida spreads antivaccine disinformation disguised as an epidemiological “study”
On Friday, Florida State Surgeon General Dr. Joseph Ladapo released a non-peer-reviewed "study" that recommends against males aged 18 to 39 receiving mRNA COVID-19 vaccines based on bad epidemiology and science. This is the first time that we've seen a state government weaponize bad science to spread antivaccine disinformation as official policy, a dangerous new escalation in antivaccine propaganda.

“Censorship” and “thoughtcrime”? Antivaxxers and COVID-19 contrarians attack California AB 2098
One of the oldest tropes favored by quacks of all stripes, including antivaxxers, is to portray any attempt at regulating their quackery as an assault on freedom of speech. It's therefore not surprising that after its passage by the California legislature prominent spreaders of COVID-19 misinformation are labeling AB2098, which seeks clarify and codify the power of the Medical Board of California...

Dr. Vinay Prasad echoes a common antivax trope that portrays concern about a deadly disease as irrational fear
Before the pandemic, antivaxxers likened concern about childhood diseases to mental illness. In the age of COVID-19, Dr. Vinay Prasad accuses medicine of "legitimizing" irrational anxiety and says we should treat COVID like the flu—with one telling omission. In a recent paid Substack, he doubles down and accuses physicians and scientists of anxiety disorders that "interfere with people's lives".
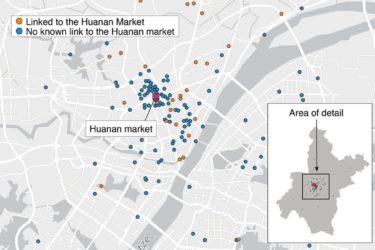
The rise and fall of the lab leak hypothesis for the origin of SARS-CoV-2
Two new studies were published last week that strongly support a natural zoonotic origin for COVID-19 centered at the wet market in Wuhan, China. Naturally, lab leak proponents soberly considered this new evidence and thought about changing their minds. Just kidding! They doubled down on the conspiracy mongering, because of course they did.
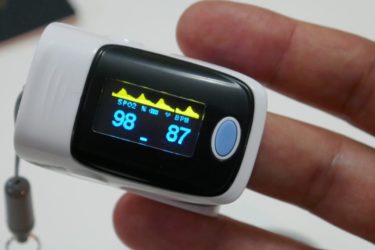
COVID-19 puts the spotlight on an unexpected racial disparity in health care
Evidence increasingly suggests that pulse oximeters, the little finger clips that measure blood oxygen, overestimate the blood oxygenation in Black patients. It's a problem that's been discussed a long time that took a pandemic to bring to public consciousness. How can SBM decrease or eliminate such healthcare disparities?

In What Is a Woman?, Matt Walsh asks a question, but doesn’t like the answers
Matt Walsh's documentary asks What Is a Woman? Unfortunately, his documentary is every bit as much of a science denying propaganda film disguised as a documentary as antivax films like VAXXED or the anti-evolution film Expelled!, and such films tend to be potent messaging tools.

Legislative alchemy and abortion: How the fall of Roe v. Wade will degrade science-based medicine
The U.S. Supreme Court overturned Roe v. Wade on Friday, eliminating the federal right to an abortion. What does that mean for science-based reproductive health and science-based medicine in general? Hint: It's not good, even for areas of medicine outside of reproductive health.

Gender-affirming care is not “experimental”
Gender-affirming care remains the evidence-based standard of care for gender dysphoria in transgender adolescents, despite claims by some laws and lawmakers that it is “experimental”.
Medical debt vs. universal health insurance: The interface between SBM and policy
This blog has long argued that the best medicine is science-based medicine (SBM). The problem is that in the US SBM is often not accessible, except at ruinous cost, which is why I argue that we have to broaden our definition of SBM to include the systems that deliver it and pay for it.