Category: Science and Medicine

Dr. Adam Cifu: “We Now Need to Accept That This is Here to Get Infected With Again and Again.”
If Dr. Adam Cifu were genuinely concerned about vaccine hesitancy, I would strongly encourage him to learn about the anti-vaccine movement. He'll discover that it is fueled not by a lack of "robust data" on COVID boosters, but rather by exactly the sort of mistrust and anti-vaccine misinformation spread by his own blog and collaborators.

Black mold is the new Candida
How concerned should we be about mold in our homes and environment?
Hospital Measures Prevented COVID Transmission
A new study finds that hospital measures to limit the spread of COVID-19 worked, and we probably should keep them.

Boosting. What To Do.
The immune system can't be "boosted." It is an inane concept used by those who promote unscientific approaches to medicine.

A good journal breaks bad: AAP spreads misinformation about glyphosate
The latest report from the American Academy of Pediatrics is filled with misinformation and missing key articles that support the well-researched conclusion that there is no legitimate evidence of negative health effects of glyphosate.

Dr. Lucy McBride: “As physicians, dispensing false hope is dangerous & unethical.”
The many sheltered physicians like Dr. Lucy McBride who confidently said herd immunity was at hand and fear of COVID was pathological are the last people who should be sanctimoniously sermonizing about the importance of trust in medicine.

The Wellness Company: How antivaccine grift becomes plain old quackery
The Wellness Company, promoted by Dr. Peter McCullough, is the product of a trend in which antivax doctors have predictably become just quacks. At least in this case, there is an amusing quack fight at the heart of it all.

A Bit of Good News: Kids Appear to Have Lower Risk of Post-COVID Conditions Than Previously Thought
Based on a recently published study using improved criteria for determining long term COVID-related health problems, it looks like kids are less likely to be negatively impacted than previously thought.
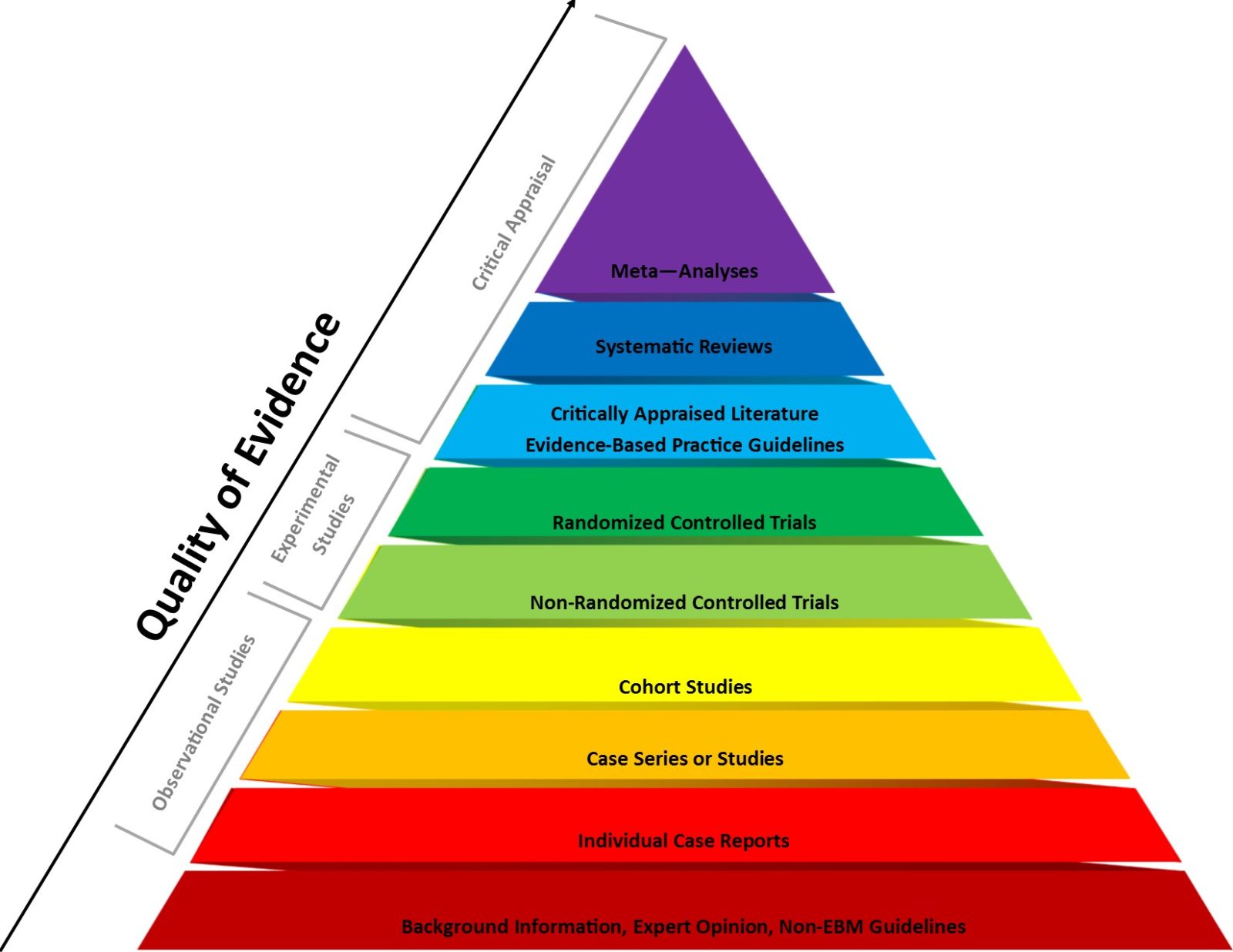
2023: The year that the evidence-based medicine (EBM) paradigm was weaponized against vaccines and public health
Evidence-based medicine (EBM) has been a very useful paradigm for assessing evidence in medicine. However, like any other framework, it can be misused, particularly when fundamentalist EBM methodolatry leads to its inappropriate application to questions for which it is ill-suited, a misuse that has been weaponized against public health during the pandemic.

Skeptics in the Pub. Cholera Chapter 6b
There were at least a dozen tents devoted to the Cholera. There was a small tent occupied by a single woman who sat at a desk with several piles of brochures. Looking closely, I could see the brochures were advertising for the Medical Societies, one stack for each of the five Societies. “Hello,” said the woman at the table. “How are you...

