“Safe and natural.” It’s a marketing phrase attached to dietary supplements that’s often accepted as self-evident. The marketing works. Supplements have a strong health halo. But evidence suggests that this reputation may be undeserved. Not only are there continued questions about whether most supplements have any health benefits whatsoever, there is also evidence that they can be harmful. We can’t even be confident that what’s on the label is actually in the bottle. Just two days ago I was notified of another long list of supplements and remedies that the FDA had identified that were contaminated with prescription drugs. These warnings about products sold as supplements appear regularly. Some time ago I asked, “What’s in your supplement?“, and noted that contamination and poor product quality standards continue to raise questions about whether supplements can be used safely at all, because the harms, when they occur, can be catastrophic. No matter how you feel about their efficacy, we can probably all agree no consumer should lose an organ from taking a health supplement. But it can happen. Like any other drug products, supplements have chemical ingredients that, once absorbed, are processed (metabolized) by the body. Our liver’s an amazing organ. Millions of years of evolution have given us the capacity to metabolize and detoxify pretty much everything we can eat. But sometimes we’ll encounter something that our liver can’t respond to. And sometimes those products cause organ damage, in the form of liver failure. It may be the labelled ingredient, or it could be something else – a contaminant that causes the harm. Unless there’s a lot of cases, it may be difficult to spot, or link harms to a specific product.
Supplements are popular – and growing in their popularity. We’re in the midst of a 40-year trend of increasing supplement use that shows no likelihood of slowing down. This is big business, a $30-billion-per-year (USD, 2011) industry with little regulatory oversight. Importantly, dietary supplements are not held and inspected to the same manufacturing and quality standards as pharmaceutical drugs – hence the persistent and worrying questions about their quality. The driver of this loose regulation is the legislation called DSHEA, which removed the onus of demonstrating safety and efficacy from the manufacturer, and put the requirement to demonstrate harm on the FDA. Yet calling a product a supplement doesn’t make it harmless. Those ingredients can harm, just like drug products. Do they deserve the same regulation? I’d argue yes – we should not have two different efficacy and safety standards, just because something is “natural” or deemed a “supplement”. The supplement industry would disagree. They may cite a lack of evidence of harms. And from one perspective, that is true. Despite their widespread use, surveillance of supplements (the limited amount that occurs) reveals generally few adverse events. It could be that many products simply don’t do anything at all – either positive or negative.
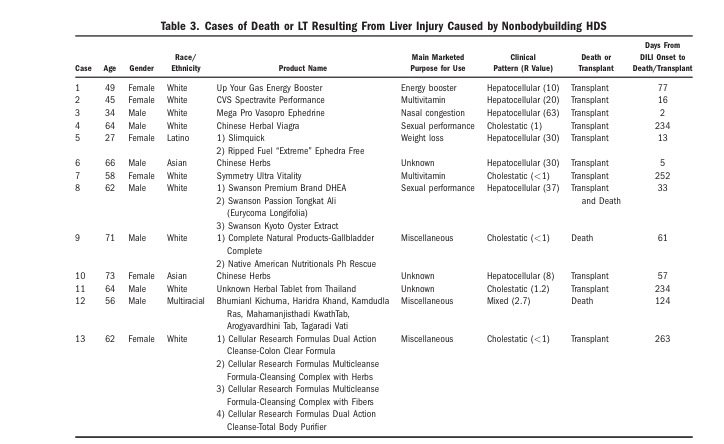
Now a new analysis from the US-based Drug-Induced Liver Injury Network gives some cautionary statistics on the harms related to dietary supplements. The study looked at data from eight referral centres, and captured data on any case of liver injury attributed to herbal and dietary supplements. Importantly, this analysis excluded cases of liver injury from acetaminophen (Tylenol). Acetaminophen poisonings, whether intentional or not, cause harms that dwarf anything else – even dietary supplements. In the USA, poisonings from this drug alone result in 56,000 emergency room visits, 26,000 hospitalizations, and 458 deaths per year. [PDF] Acetaminophen is responsible for more overdoses, and overdose deaths [PDF], than any other pharmaceutical product. In this latest report, patients were followed if they had documented liver toxicity that was suspected as being either medication- or supplement-related. A standardized protocol was used to determine the relationship and assess causality. Cases were only included if the likelihood of a causal relationship was at least probable. Other causes of liver damage were ruled out. Rates of hospitalization, and outcomes like liver transplant or death were also followed. The analysis divided the cases into two groups: those with injuries attributed to bodybuilding supplements, and those from other supplements. Given the statistics, one of the best ways to avoid liver damage from supplements seems to be to avoid anything related to bodybuilding, as they are the most common cause of supplement-induced liver damage. These injuries occur predominantly in younger men and they tend (according to the authors) to cause a similar injury: prolonged jaundice and eventual recovery. Liver injury caused by other supplements is more varied, and has different outcomes, including a greater risk of liver transplant or death.
Between 2004 and 2013 the researchers found 839 cases of liver injury eligible for consideration: 709 were from drugs, and 130 from supplements. In the pool of 130 injuries linked to supplements, 45 (35%) were from bodybuilding supplements and 85 (65%) were from other supplements. The analysis found no patient that took bodybuilding supplements died or required a liver transplant. In the non-bodybuilding group, 13 patients died or required a transplant. Interestingly, the severity of the reaction to supplements was greater than the liver toxicity from conventional meds, though conventional med reactions were associated with a greater risk of death (7% vs. 4%, respectively.) Here’s the list of the products that caused death or led to a liver transplant, and the full list of all implicated supplements may be downloaded here:
What’s surprising? There’s a lot of products on the full list that should not be associated with liver problems at all, like multivitamins. It’s highly unlikely that a product like a multivitamin would cause liver damage. What’s also possible (and probably more likely) is product contamination. The authors note that the pattern of liver damage associated with bodybuilding products is consistent with what might be expected if the products were contaminated with anabolic steroids. But we don’t know for sure – to truly identify the cause, it would be necessary to assay and test every single product associated with liver damage, in order to determine the actual ingredients and the most likely cause. In this survey, 217 products were associated with the 130 cases of liver injury. Amazingly and frighteningly, only about 80% had identifiable ingredients according to the label. And given the product quality concerns that continue to plague the supplement industry, determining which products and ingredients are the real risk factors is all-but impossible, as we can’t trust the labels to be accurate.
Tip of the harms iceberg?
Liver damage and liver failure is both catastrophic and fairly easily identified. Other harms may not be immediately attributed to supplements, which may mean that harms from supplements may be under-reported. I’ve mentioned several factors before, which include:
- The belief that supplements are harmless, accentuated by beliefs that “natural” substances don’t harm, so the link is never suspected.
- The lack of routine health professional monitoring.
- The reluctance to disclose harms and side effects to a health professional, in part because they may not want to disclose they’re taking a supplement at all.
- Not knowing that adverse events should be reported, or how to report them.
- Use may be driven through consultation with “alternative” medicine providers, and those experiencing harm may be reluctant to report experiences to “conventional” health providers like physicians or pharmacists.
- Concern about the risk of additional regulation, leading to a reluctance to report harms.
- Supplements may be used short intervals for self-limiting conditions, and long-term harms may not appear (or be detectable).
Reducing the risk
If you don’t look for it, you won’t find it. And if you set the system up to minimize the likelihood of finding harm, it should be no surprise that supplements are not associated with a lot of safety concerns or reports. Given the way this data was assembled, it’s not possible to estimate a relative risk of harm, or even assess the any change in incidence of liver damage associated with supplements. But it should put to rest any claims that supplements are without harm. Even more troubling, it doesn’t look like there’s any way to avoid the risk of supplement harms, unless you decide to avoid them completely. There’s no question that drugs can cause liver problems, and they do so with more frequency than supplements. But these harms are not related to widespread product contamination – a risk that is very real, and difficult to avoid, with any dietary supplements sold today. Until regulations are tightened, I don’t expect manufacturers to do much to improve the quality of their products. Which is unfortunate, because until we can consistently trust what’s on the label, it’s going to continue to be hard to accept dietary supplements as either safe or effective.
Reference
Navarro V.J., Herbert L. Bonkovsky, Timothy Davern, Robert J. Fontana, Lafaine Grant, K. Rajender Reddy, Leonard B. Seeff, Jose Serrano, Averell H. Sherker & Andrew Stolz & (2014). Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network, Hepatology, n/a-n/a. DOI: http://dx.doi.org/10.1002/hep.27317