Tag: dichloroacetate
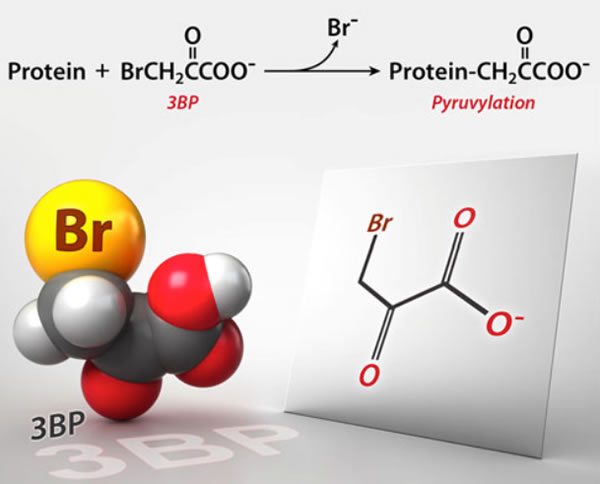
3-Bromopyruvate: The latest cancer cure “they” don’t want you to know about
Why is it that whenever naturopaths and other quacks embrace a new "cancer cure," somehow "they" (whoever "they" are) don't want you to know about it? In this case, it's 3-BP, an actual experimental drug that shows some promise but is by no means ready for prime time (or FDA approval) yet.
The latest chapter in the seemingly never-ending saga of dichloroacetate as a cancer treatment
The road from an idea to a useful drug is a long one, and in cancer it is often particularly long. One reason is that to be able to tell whether a given treatment is effective against cancer often takes several years at a minimum, in order to determine if patients receiving the new treatment are surviving their disease longer than those...

