Tag: Patient Safety

An Update on FDA Concerns Over Homeopathic Teething Products
Steven Novella recently wrote a post discussing an FDA warning against the use of homeopathic teething products over safety concerns related to the possibility of toxic amounts of belladonna. He goes into the hypocrisy of the FDA regulation of homeopathic products, a topic covered numerous times here on Science-Based Medicine, as well as the misleading initial response from Hyland’s, producers of the...

FDA Warns About Homeopathic Teething Products
The FDA recently put out a consumer warning about homeopathic teething gels and pills. The warning states: The FDA recommends that consumers stop using these products and dispose of any in their possession. The warning is not because all homeopathic products are inherently useless. As we have discussed here often, the basic principles of homeopathy are pure pseudoscience. The practice of diluting...
Holding the supplement industry to account: Can we learn from tobacco regulation?
A new paper compares the supplement industry to Big Tobacco and argues that states should use the same tactics to improve consumer safety and protection.
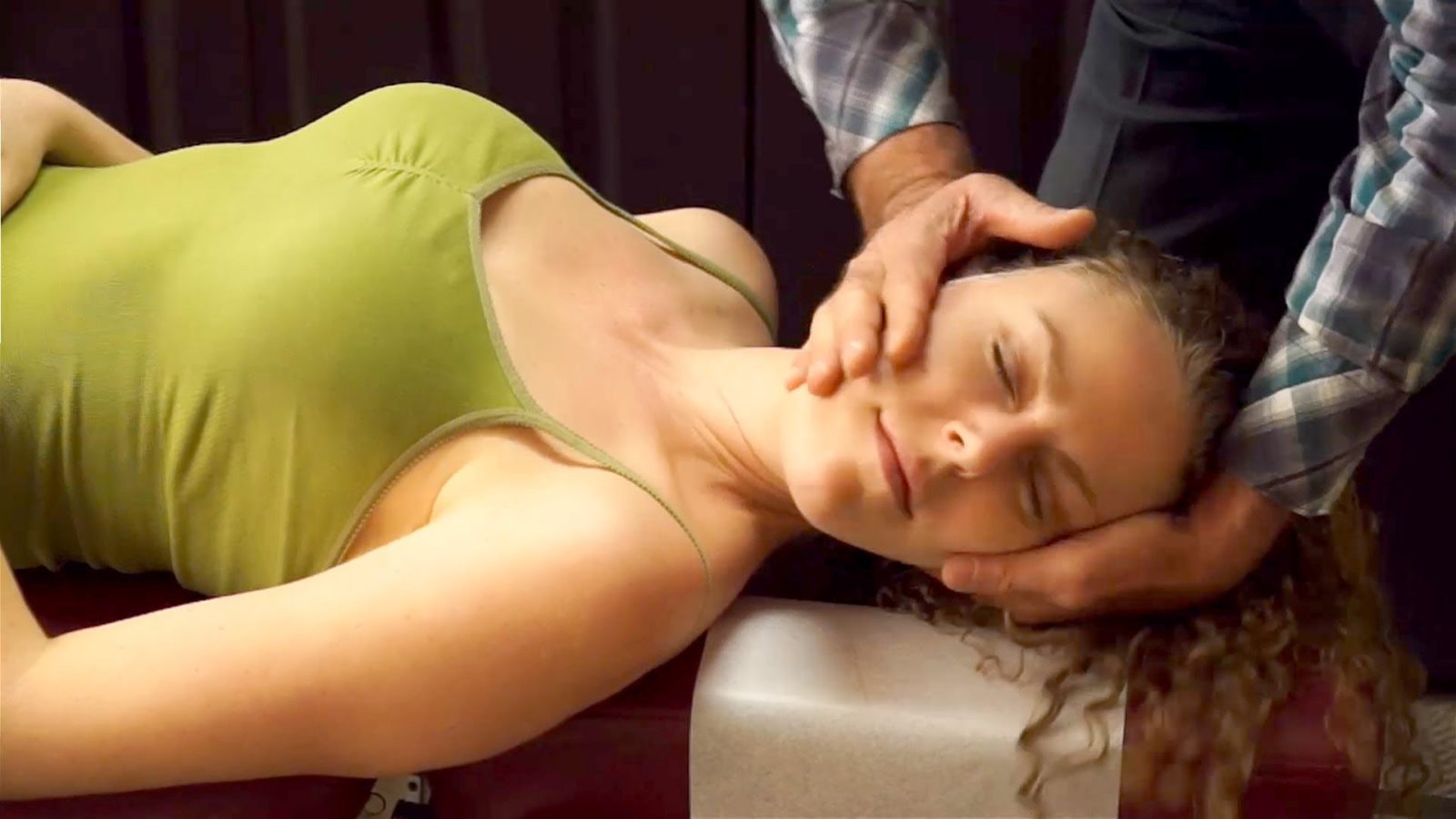
Chiropractic and Stroke: The question is not answered
I am off to Chicago for 5 days to wow the SMACC crowd with my ID/SBM acumen. I hope. Given that most of my multiple-personalities do not seem to be able to get any work done, I am forced to write a brief post this week, limited by the battery life on my MacBook Air. Whatever I get down on paper? pixels?...
Double-Talk And Paternalism
One of the more frustrating things about practitioners who promote unsafe and scientifically discredited medical practices is their tendency to change their message for different audiences. One day they’ll tell you that they espouse only evidence-based practices and the next they’ll be promoting snake oil. This double talk is hard to combat, since to disprove them one would essentially have to provide...