Recently ProPublica and This American Life (TAL) released the results of an investigation into acetaminophen, the active ingredient in Tylenol. TAL devoted an entire episode to the issue, and ProPublica has published several stories on acetaminophen’s toxicity, how it can cause harm, and how it is regulated.
The investigation summarizes the key “Takeaways” as follows:
- 150 Americans die per year from accidental acetaminophen overdoses
- The safety margin (safe dose vs. toxic dose) with acetaminophen is small
- Both the FDA and the manufacturer, McNeil, have known about the toxicity for years
- For over 30 years the FDA has failed to implement measures to reduce the risk of harms it knew existed
- The manufacturer has taken steps to protect consumers but has also opposed other safety measures
While Tylenol is a single brand out of hundreds of prescription and non-prescription products that contain acetaminophen as an active ingredient, it is the brand most closely associated with the chemical. Amazingly for a drug that has no patent and lots of competition, Tylenol products are estimated to make up half of all non-prescription acetaminophen sales in the US, a testament to the power and effectiveness of marketing. (It’s also a clear refutation to alt-med arguments that unpatented products can’t be profitable, or aren’t of interest to the pharmaceutical industry.) While much of the focus of the investigation centers on the corporate behavior of Tylenol’s manufacturer, McNeil, (a division of Johnson & Johnson), it is important to keep in mind that no single company is responsible for acetaminophen sales and marketing.
Before diving into the investigation and its findings, a review of the pharmacology and toxicity of acetaminophen will help with the context. (More detail, if you’re interested, is contained in a prior post.) Acetaminophen is a drug that has analgesic (pain relief) and antipyretic (fever-reduction) actions. Unlike non-steroidal anti-inflammatory drugs (NSAIDs) like acetylsalicylic acid (ASA, aspirin), ibuprofen (Advil) and naproxen (Aleve), acetaminophen has no effect on prostaglandin synthesis – so it is not an anti-inflammatory. This lack of anti-inflammatory action gives it a completely different side effects profile. Unlike NSAIDs, acetaminophen is unlikely to cause gastrointestinal ulcers, blood disorders, or kidney problems, all common with NSAIDs. Nor does acetaminophen increase the risk of cardiovascular events such as heart attacks and strokes, like some NSAIDs. Compared to NSAIDs, acetaminophen also has few interactions with other drugs, so it can safely be used for pain relief in those on multiple medications, or with other medical conditions where other pain relievers might have unwanted effects. Acetaminophen is not a narcotic, and provides a non-addictive alternative for managing chronic pain issues. It can also be used during pregnancy, and while breastfeeding. Finally, at the recommended dose, acetaminophen is almost completely free of significant side effects. This can be contrasted with NSAIDs, that cause a considerable number of drug-related hospitalizations each year. The problem with acetaminophen appears when an excessive dose is taken. And in that situation, acetaminophen is much worse than any NSAID.
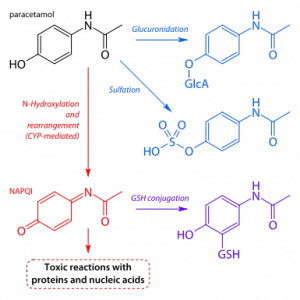
The body eliminates acetaminophen through the action of liver enzymes that converts the base drug into substances (metabolites) that are easier to for the kidneys to filter and excrete. In overdose situations, this system fails catastrophically. We are physiologically able to safely metabolize a small amount of acetaminophen at a time – about 4,000mg in a 24 hour period for adults is generally considered the safe upper limit. The two main metabolic paths are sulfation and glucuronidation. These paths can be exhausted and overwhelmed in overdose situations, which is when other enzymes take over. N-hydroxylation creates a chemical compound that causes liver damage. In the diagram below: blue is good, and red is bad (note, paracetamol is acetaminophen):
The toxicity of acetaminophen has been recognized since at least 1966. As I’ve discussed before, acetaminophen poisonings are a significant public health issue. The acetaminophen poisoning antidote is a universal feature of emergency rooms – overdoses are that common. The raw numbers are huge: poisonings from this drug alone result in 56,000 emergency room visits, 26,000 hospitalizations, and 458 deaths per year. [PDF] This makes acetaminophen responsible for more overdoses, and overdose deaths, [PDF] and acute liver failure, than any other drug product.
Poisoning from acetaminophen will usually take one of two forms:
- the one-time ingestion of a massive dose (e.g., a suicide attempt)
- the prolonged ingestion of a dose that exceeds the body’s ability to eliminate the drug safely
Between the two, massive single overdoses are much easier to manage medically. An antidote (acetylcysteine) does exist, which can save an individual’s liver, and their life, if given within 8 hours of an overdose. Poisoning due to chronic ingestion, which are often unintentional, are far more difficult to manage. The early signs of liver damage are not obvious, and can mimic flu-like symptoms. Once hepatic damage has started, the antidote’s effectiveness drops. In severe cases, a liver transplant is required.
It’s the second form of overdose, the inadvertent poisonings, that are understandably the focus of the ProPublica/TAL investigation. Let’s examine the “takeaways” in order. The first is the number of acetaminophen deaths, which they claim at 150 per year in the USA. While The Poison Review disagrees with the show’s methodology and its fatality calculations, the FDA’s own estimate was 458 deaths (from all causes) per year in the period 1990-1998. Case reports collected over the past several decades attest to the fact that unintentional poisonings do occur regularly, although the distinction between deliberate and inadvertent overdoses isn’t always clear, especially when the poisoning is fatal. So while there is some legitimate controversy over the actual numbers, there is no question that accidental poisonings do occur.
TAL/ProPublica’s second “takeaway” is that acetaminophen has a narrow safety margin. Again, it is well established that massive overdoses are fatal unless treated promptly. What is less clear is when liver toxicity becomes an issue with acetaminophen use. The normal maximum dose for adults is 4,000mg per day, and toxicity is felt to be unlikely from a single dose of 7.5-10g. What’s not clear is whether modestly but consistently exceeding the 4,000mg/day maximum is likely to cause harm, or if 4,000mg/day may be too much for some. Case reports suggest that even repeated daily doses of 5,000mg/day to 7,500mg/day have been associated with liver damage. That’s obviously concerning, as modestly exceeding the maximum dose could easily and inadvertently occur from ignoring the dosing instructions, calculating the wrong dosage (particularly in children), or taking two products that both contain acetaminophen.
Acetaminophen is unquestionably toxic in overdose, and potentially harmful at or above the maximum recommended dose. So does that mean that NSAIDs are always preferable? Not necessarily. Massive overdoses of anti-inflammatory drugs are, on balance, less harmful than acetaminophen. Deaths from acute overdose of NSAIDs are rare. What is more insidious and problematic with NSAIDs is the side effect profile I described above. What’s worse, NSAIDS have the potential to cause significant harm, even when dosed appropriately. The Propublica/TAL reports don’t elaborate on the full toxicity profile of the NSAIDs, which implies they are safer – a potentially misleading comparison. Comparing acetaminophen’s toxicity with NSAIDs isn’t an apples-to-apples comparison, and it can’t be quantified. Which is worse in overdose? Acetaminophen. What’s more likely to cause harm at normal doses? NSAIDs. Which is the safest for any given person? It depends. This is the crux of the issue, and I’ll come back to this later. Considering 28 billion doses of acetaminophen were consumed in 2005, this suggests the overwhelming majority of acetaminophen users are not encountering toxicity and poisoning problems with their use.
This isn’t to say that we should accept 150-400+ deaths per year as an acceptable cost of making a drug available, if there are ways we can minimize overdoses, especially the unintentional ones. The third “takeway”, that the FDA has been aware of this issue for years, should not be a surprise. The toxicity of acetaminophen has been understood since the 1960s by health professionals and regulators alike. The population-level harms are well documented in the medical literature. What’s relevant is whether the regulator acted appropriately to address this information. This is the investigation’s fourth takeaway, and where the most effective points in the investigation are made. The FDA has been discussing various measures to enhance various aspects of acetaminophen’s labeling for an astonishing 30 years, with very little to show for it. Recommendations have been drafted, formal advice has been provided, and the FDA has repeatedly deferred making substantive labeling, packaging, or regulatory changes that have the potential to reduce the risk of accidental poisoning. The investigation does a good job describing some of the major changes recommended, and why they are warranted.
The TAL episode describes the tragic death of an infant who died of an overdose due to a dosing error, caused by confusion between different brands of Tylenol liquid. Two formulations for pediatric use have traditionally been available – a concentrated version for infants, dispensed with a dropper, and a less concentrated version for children, dispensed with a spoon or oral syringe. In the case described, the parents were given dosing instructions for Tylenol based on the children’s version of the drug. The parents gave the same volume of the infant formula, resulting in an overdose. The baby suffered liver failure and eventually died of acetaminophen poisoning. This tragic, preventable death sets up TAL and Propublica for a discussion of both manufacturer and regulator behavior, and how the actions (and inactions) of both parties may have been factors in these deaths.
The FDA tightly regulates what drug manufacturers can say about the drugs they sell. Before any manufacturer of over-the-counter drugs can change the dosing instructions on its label, it must obtain FDA approval. And that approval can take years, TAL/Propublica discovered. Unless you’ve ever tried to give Tylenol to an infant, you may not know that Tylenol products sold in the USA do not contain instructions for dosing children under the age of two. The instructions indicate that consultation with a physician is required. There’s no therapeutic reason for this – dosing instructions for this age group are listed on Tylenol bottles sold in Canada, for example. McNeil has been asking the FDA to approve a label change for years, arguing that specific instructions will reduce the likelihood of simply guessing the dose, potentially raising the risk of overdose. The FDA has just recently agreed, and this label change is coming, which should reduce the risk of wrong doses.
One step that McNeil could have done, TAL/Propublica argues, would have been for McNeil to remove one version of its Tylenol liquid, which would eliminate the risk that one product could be accidentally substituted for each other. But McNeil didn’t act. A recent change approved by the FDA will mean the two products will continue to exist – but at the identical concentration, so substitutions won’t carry the same risk. (Interestingly, both products, at two different concentrations, continue to be sold in Canada.) This isn’t the only issue the investigation finds with McNeil. The website includes a much longer list of McNeil’s actions that appear to put business interests ahead of safe use: It has repeatedly fought labeling changes that would put it at a competitive disadvantage to its competitors in the pain reliever marketplace. It fought the FDA’s modest public service campaign to warn about the risks of acetaminophen overdose. And it never disclosed to the FDA its own (ultimately abandoned) work to develop a safer formulation of acetaminophen.
In fairness to McNeil however, it needs to be reiterated that Tylenol is just one brand of acetaminophen liquid among hundreds of acetaminophen products on the market. Without the FDA compelling the entire market to change its labeling or packaging, it’s unlikely that a single manufacturer (albeit the biggest) would act alone. And it’s not clear that different manufacturers, each selling their own concentrations of acetaminophen liquid, would actually create a safer marketplace for consumers. For these types of changes to be truly effective, there needs to be a coordinated approach that applies to all manufacturers – one that only the FDA could have mandated. But it didn’t, for years.
So what are we ultimately left with? Balancing access against patient safety has no single correct answer. There are no risk-free strategies – even shifting use of acetaminophen to substitute drugs, like NSAIDs, could have unforeseen negative consequences. The Propublica/TAL investigation succeeds in raising the toxicity profile of acetaminophen among the public. I hope it will encourage consumers to follow the directions carefully, and to double-check the ingredients and doses of the acetaminophen products they use. It has also shone a bright light on the FDA’s regulatory inertia, which may compel more decisive and timely action to improve labeling, which may in turn reduce accidental overdoses. Where the investigation is weaker is in describing the relative risk of acetaminophen compared with its substitutes, which may leave consumers with the erroneous impression that NSAIDs are safer and more appropriate for pain relief and fever reduction. My personal take is that acetaminophen, when used appropriately, is an exceptionally safe medication, with several substantial advantages over NSAIDs. But I also agree that there’s a lot more that can and should be done to improve the public’s capacity to use this medication safely. Ultimately as a health professional I’m an advocate for appropriate regulation and marketing that gives consumers the information they need to make informed and safe decisions about their own care.