If science-based medicine reflects the application of the best evidence, then we should expect practices to change when new data emerges. In the long run that’s generally true, and the progressive gains we’ve seen in the management of disease reflect this. But in the short run, change can be maddeningly slow, and there are many areas of medicine where we could be doing a better job of applying what we already know to improve outcomes and reduce harms. One area where this is obvious is drug treatments, which can provide remarkable benefits but are also sources of significant harms.
Somewhat problematically, the real world is often the setting where the full extent of harms from treatments are identified. Bringing new drugs to market means tradeoffs: Do you demand larger and longer clinical trials to get as much information as possible about a drug before it’s sold? Or do you approve based on more preliminary, potentially weaker evidence, to meet (potentially) important patient need? There is no set formula or right answer to this questions – it’s ultimately a value judgement exercised by regulators like the FDA, who decide which drugs are allowed for sale (the benefits are assumed, overall, to exceed the harms) or removed for sale (when the opposite is felt to be the case).
Once a decision is made to allow a drug for sale, the evidence on risk and benefit continues to emerge, sometimes from continued clinical trials, possibly from adverse drug reactions, and occasionally from epidemiological studies that are conducted to better understand the overall safety and efficacy of treatments. In general, once a drug is on the market the threshold for removing it is fairly high. For example, I suspect a drug that taken in overdose can cause liver failure and is the leading cause of liver transplants would probably not be approved if developed today, even if acetaminophen (paracetamol, or APAP) is an effective pain reliever and safe at appropriate doses.
Tylenol is far from the only drug with significant benefits as well as significant harms. Nowhere are trade-offs between risks and benefits more apparent than with the non-steroidal anti-inflammatory drugs (NSAIDs). Some, like ibuprofen (Advil, Motrin), ASA (Aspirin), and naproxen (Naprosyn, Aleve) are available without prescriptions in some countries, while about a dozen more including celecoxib (Celebrex) and diclofenac (Voltaren) are usually prescription-only.
NSAIDs are among the most widely used drugs, starting in infancy for pain and fever, right through to the elderly where they are standards for treating osteoarthritis and other muscle and skeletal conditions. NSAIDs all work in the same way, blocking the cyclooxygenase (COX) enzyme, responsible for the production of prostaglandin messenger substances that cause pain, inflammation and fever. The mechanism of action is also responsible for the extensive side effect list, a consequence of COX enzymes being distributed throughout the body. Ulcers are the most well-known effect and hospitalization secondary to gastrointestinal bleeding from NSAIDs is common. Fortunately these side effects can be prevented with drugs like proton pump inhibitors. The other well-known side effect is cardiovascular disease, and NSAIDs seem to increase the risks of heart attacks and strokes. There are two main subtypes of COX enzymes (conveniently, COX-1 and COX-2) and the affinity of a particular NSAID for the different COX enzymes seems to be the main factor influencing the degree of cardiovascular risk. The COX-2 inhibitors like Vioxx had less effects on COX-1 in the gastrointestinal tract, reducing side effects, but effects on COX-2 were linked with increases in events like heart attacks and strokes. Importantly, other NSAIDs interfere with the beneficial effects of ASA (aspirin) on platelets that can give protective effects against cardiovascular disease.
Now in a world that responds quickly to new evidence we’d expect to see a shift away from the NSAIDs with the worst side effect profiles and towards more use of those with the best side effect profiles. After all, no NSAID has been shown to be clinically more effective than another in trials, although individual responses can vary. But the most toxic NSAIDs are still used widely, as was noted recently in a paper published in PLoS Medicine, entitled, Use of Non-Steroidal Anti-Inflammatory Drugs That Elevate Cardiovascular Risk: An Examination of Sales and Essential Medicines Lists in Low-, Middle-, and High-Income Countries by Patricia McGettigan and David Henry. NPR’s headline was perhaps a more succinct summary of the key finding, which was World’s Most Popular Painkiller Raises Heart Attack Risk. It’s important to note that this paper doesn’t present any previously unpublished information about NSAID safety, but it does an effective job of illustrating the disconnect between medical evidence and medical practice.
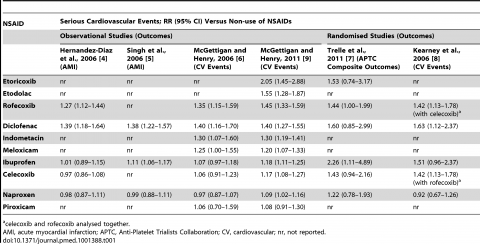
The authors start by summarizing the known risks of cardiovascular events with NSAIDs in a handy table:
There’s a lot here. The columns summarize the risks of NSAIDs identified by trials, and the rows show the different drugs studied. The relative risk is reported – that is the degree to which the baseline (or underlying) cardiovascular risks were observed to increase as a result of that particular NSAID. Rofecoxib (Vioxx), now withdrawn, has a clear risk of 1.27 to 1.45 x versus non-users. What’s remarkable is what the NPR headline hints at. The risks from diclofenac, the most popular NSAID in the world, is at least as high as Vioxx: A 1.39-1.69 relative risk. That is, cardiovascular events are 40-70x more likely among users of diclofenac compared to non-users.
Let’s put the relative risk in context. The actual increase in risk to anyone is dependent on the baseline risk. A young, otherwise healthy make or female would have a small underlying risk of a cardiovascular event. So the incremental risk from taking any NSAID, even one like Vioxx, is still tiny. But the risk changes in patients with multiple medical conditions who are already at high risk of heart attack or stroke. A relative risk increase of 40% could imply a significant increase in the absolute risk. So the relevance of this controversial debate about NSAIDs really depends on the underlying risk.
So if diclofenac is the worst, which NSAID is best? The data suggest the risk with naproxen is similar to non-users of NSAIDs. Why naproxen is the least toxic is unclear, but it may be because of its high affinity for COX-1 which may give it antiplatelet effects that are actually beneficial. The data are pretty consistent – if you’re worried about minimizing cardiovascular risk, naproxen looks like a much safer choice than the other NSAIDs. Beyond naproxen, celecoxib (Celebrex) and ibuprofen have a dose-dependent elevation in relative risk that does not seem to be significant at the lower doses typically used.
The second part of the paper is an examination of the worldwide use of NSAIDs, based on a basket of countries representing a range of incomes. Countries studied included Australia, England, Canada and thirteen Asian and South-Asian countries. The United States was not included in the survey, and there’s no explanation why. Based on an analysis of prescriptions and sales, diclofenac is the most widely used NSAID, and diclofenac plus etoricoxib (not available in North America, but with a similar risk profile to Vioxx) make up one-third of all NSAIDs used. The authors note that the World Health Organization’s recommended list of “essential” drugs still includes diclofenac, despite a risk profile that is equivalent to Vioxx.
If you’re looking for a good example of the disconnect between research and practice and the avoidable harms, this is it. Despite the risks of diclofenac being known for at least a decade, worldwide it’s still widely used and enormously popular. Whether due to slow-to-change physician prescribing or to other factors, like over-the-counter purchases, it’s clear that something is blocking the switch. The authors argue that regulators should withdraw diclofenac, a blunt instrument that would certainly reduce use. While this action would be a negative for those that have found diclofenac to be the most effective NSAID, it would almost certainly reduce the incidence of heart attacks and strokes in those countries where it’s used indiscriminately.
Topical NSAIDs
In the United States and in other countries, diclofenac is also sold as Voltaren cream or “Emulgel,” a topical product that you apply directly to the site of pain. It’s marketed for both short-term and chronic pain conditions. I admit to having dismissed topical NSAIDs as placebos when I first heard of them, believing that oral delivery was essential for getting meaningful amounts to the side of action. I was wrong. Not only do topical NSAIDs work, they are quite effective and preferable to oral NSAIDs for some conditions (especially superficial musculoskeletal problems, like arthritis or tendonitis). The main advantage of topical NSAIDs is the reduced exposure of the rest of the body to the product, which reduces the side effect profile. Given the toxicity of NSAIDs is related in part to the dose, it follows that topical treatments should have a better toxicity profile. Consequently, the cardiovascular risks of topical diclofenac, even in those with a high baseline risk of disease, should be negligible with the topical forms.
Conclusion
All NSAIDs are hazardous, but but some have higher toxicities than others. For occasional and long-term use, products like ibuprofen and naproxen are safer and as effective as other NSAIDs. Diclofenac remains a popular NSAID despite the evidence that it causes heart attacks and strokes in rates similar to that of Vioxx. There’s little rationale for allowing its continued sale, and there’s good justification for withdrawing it from the market worldwide. Until practices change or regulators act, this will remain another example of the gap between evidence and practice, a gap that’s likely resulting in thousands of avoidable deaths each year.