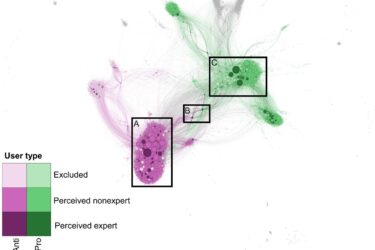
Yet more evidence that we physicians need to clean up our act
A recent study found that physicians and scientists who are perceived as "experts" are prevalent within the antivax community and more influential because of their status as physicians and scientists. Why do physicians continue to tolerate antivax quacks within our ranks?

COVID-19 antivax quacks are now “repurposing” ivermectin for cancer
A year ago, I noticed that COVID-19 quacks were touting the "repurposing" of ivermectin to treat cancer. Now, familiar COVID-19 antivaxxers—cough, cough, FLCCC—have turbocharged this quackery.

Antivax quacks are continuing to make up fantastical biological mechanisms for COVID-19 vaccine “shedding”
A couple of weeks ago, I discussed why antivax quacks' claimed biological mechanisms for COVID-19 vaccine "shedding" reminded me of homeopaths. Confabulation about fantastical scientific mechanisms continues, courtesy of "A Midwestern Doctor."
“New school” antivax goes old school as Byram Bridle asks if COVID-19 vaccines will drive an “epidemic” of autism
Wakefield redux? Antivax scientist Byram Bridle just took the "new school" antivax movement old school by implying that COVID-19 vaccines might cause an "epidemic of autism." Everything old is new again, sort of.
Why antivax arguments for COVID-19 vaccine “shedding” remind me of homeopathy
An antivaxxer by the 'nym "A Midwestern Doctor" makes an argument that COVID-19 vaccine "shedding" is not impossible despite the basic science that concludes it is. Sound familiar?

The Wellness Company: How antivaccine grift becomes plain old quackery
The Wellness Company, promoted by Dr. Peter McCullough, is the product of a trend in which antivax doctors have predictably become just quacks. At least in this case, there is an amusing quack fight at the heart of it all.
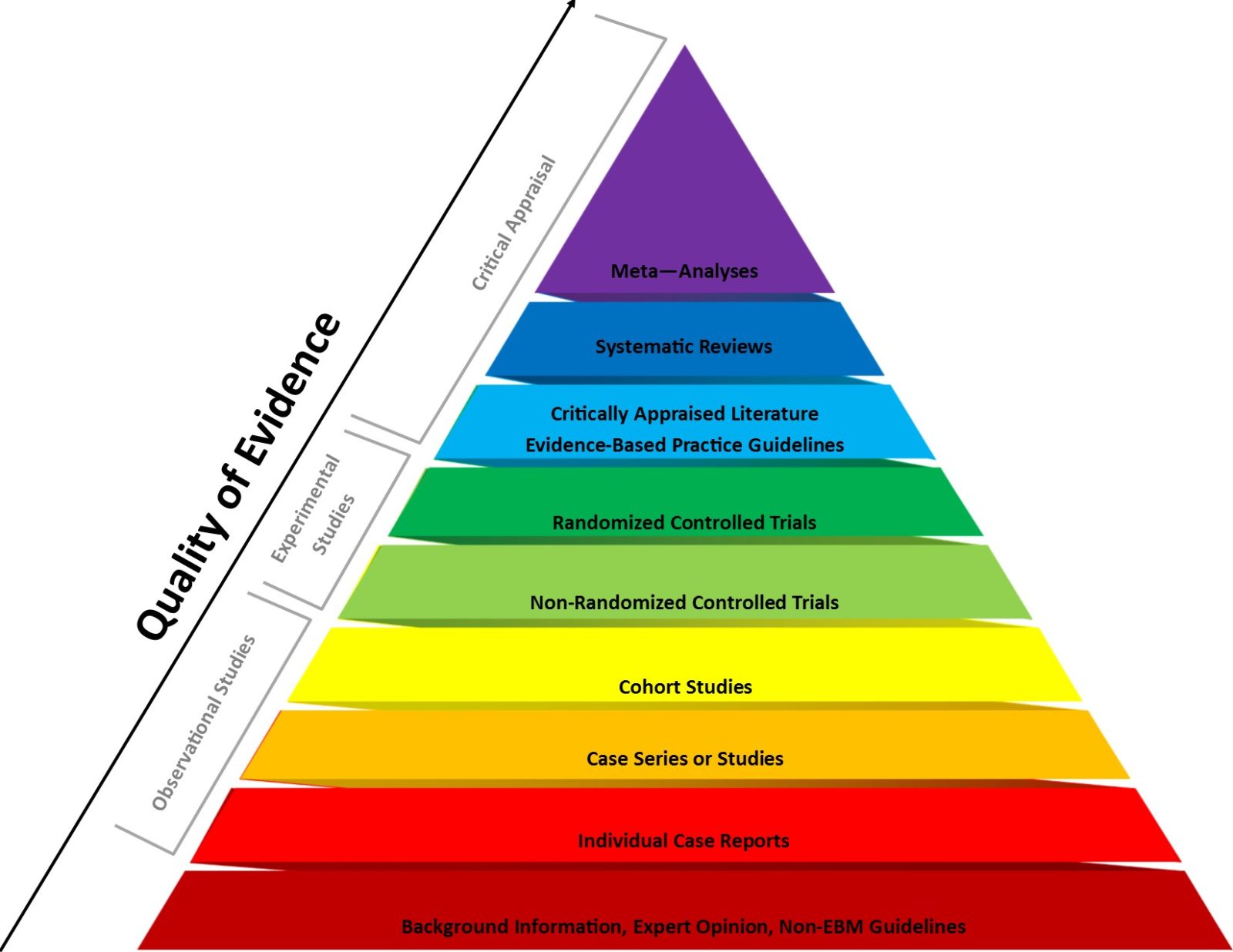
2023: The year that the evidence-based medicine (EBM) paradigm was weaponized against vaccines and public health
Evidence-based medicine (EBM) has been a very useful paradigm for assessing evidence in medicine. However, like any other framework, it can be misused, particularly when fundamentalist EBM methodolatry leads to its inappropriate application to questions for which it is ill-suited, a misuse that has been weaponized against public health during the pandemic.
RFK Jr. and his “I’m not anti-vaccine” rejoinder to being confronted with his past antivax statements: A primer
On Friday, CNN host Kasie Hunt interviewed antivax presidential candidate Robert F. Kennedy, Jr. Although she did better than most journalists confronting him for his past antivax statements in that she played a clip of one of his antivax statements, she clearly hadn't anticipated his response, which should have been very predictable given that he's been using it for at least 15...
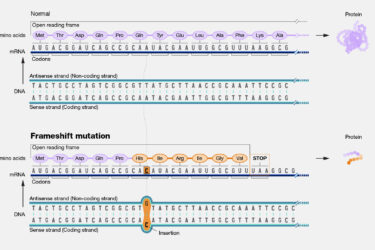
Do mRNA vaccines produce harmful “junk proteins” that “gunk up” the cell and cause unintended “off-target” immune responses?
A new study is making the rounds in the antivax crankosphere. The study found that the modified mRNA used in the Pfizer vaccine can cause a frame shift (to be explained) that results in the production of proteins besides the intended spike protein. The findings are, as you probably guessed, a big nothingburger compared to how they are being spun.


