Category: Clinical Trials

The Apple Heart Study
A recent study involving the Apple Watch raises some interesting points about modern clinical trials. It also has some implications and conclusions about screening for atrial fibrillation (a. fib).
Spinning a negative acupuncture study: Same as it ever was
Investigators at M.D. Anderson Cancer Center reported the results of a trial of acupuncture for xerostomia (dry mouth) secondary to radiation therapy for head and neck cancers. It was a negative trial, but investigators still tried to spin it as positive, but with a twist. There was a large difference between results found at M.D. Anderson and the second site in China....

Would you pay $1 million to enroll in a phase 1 clinical trial of an “anti-aging” gene therapy?
Libella Gene Therapeutics, LLC made the news last week for announcing a "pay-to-play" trial of its telomerase-based anti-aging gene therapy. What was shocking about the announcement was not that it was a "pay-to-play" trial, given that such trials have become all too common, but rather the price of enrollment: $1 million. Worse, the trial is being conducted in Colombia; the therapy doesn't...

Clínica 0-19: False hope in Monterrey for DIPG patients (Part 5, A dubious poster is presented)
Clínica 0-19 is a clinic run by Instituto de Oncología Intervencionista (IDOI) Drs. Alberto Swiller and Alberto Garcia in Monterrey Mexico that claims to have a much higher rate of survival for patients with DIPG, a deadly brain cancer, than conventional treatments. Patients come there from all over the world for an unproven concoction of chemotherapy drugs administered directly into arteries feeding...
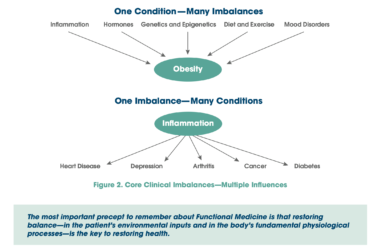
The Cleveland Clinic publishes a study claiming to show benefits from functional medicine. It doesn’t.
Last week, the Cleveland Clinic published a study purporting to show that functional medicine improves health-related quality of life. Not surprisingly, on closer examination, there's a lot less to the study than meets the eye, and its results are quite underwhelming.
“SuperMannan Cures Bladder Infections!” Really?
The ads claim SuperMannan cures bladder infections. The science is a single uncontrolled study of 9 women; its design is a recipe for disaster.
Electromagnetic healing devices for dogs: Studies show “Meh”
Does a pulsed electromagnetic field device work to help dogs recover from surgery? The answer is below. Spoiler alert: The answer is "probably not".

There’s No Vaccine for HIV/AIDS, But There’s Truvada
Science has made great strides in understanding, treating, and preventing HIV/AIDS. We can hope for an AIDS vaccine, but meanwhile there is a pill that can markedly reduce the risk of becoming infected.

Federal “right-to-try” over a year later: Still a failure and still about the money (and weakening the FDA)
Federal "right-to-try" legislation was passed and signed into law by President Trump over a year ago. Advocates promised that lots of terminally ill people who were dying then would be saved by having the right to "try" experimental therapies outside of the context of clinical trials. That has not happened. This should come as no surprise, because right-to-try was never about getting...


A world-renowned placebo researcher asks, “Does placebo research boost pseudoscience?”
Professor Fabrizio Benedetti is the most famous and almost certainly also the most influential researcher investigating the physiology of placebo effects. In a recent commentary, he asks whether placebo research is fueling quackery, as quacks co-opt its results. The answer to that question is certainly yes. A better question is: How do supporters of science counter the placebo narrative promoted by quacks,...