Category: Legal
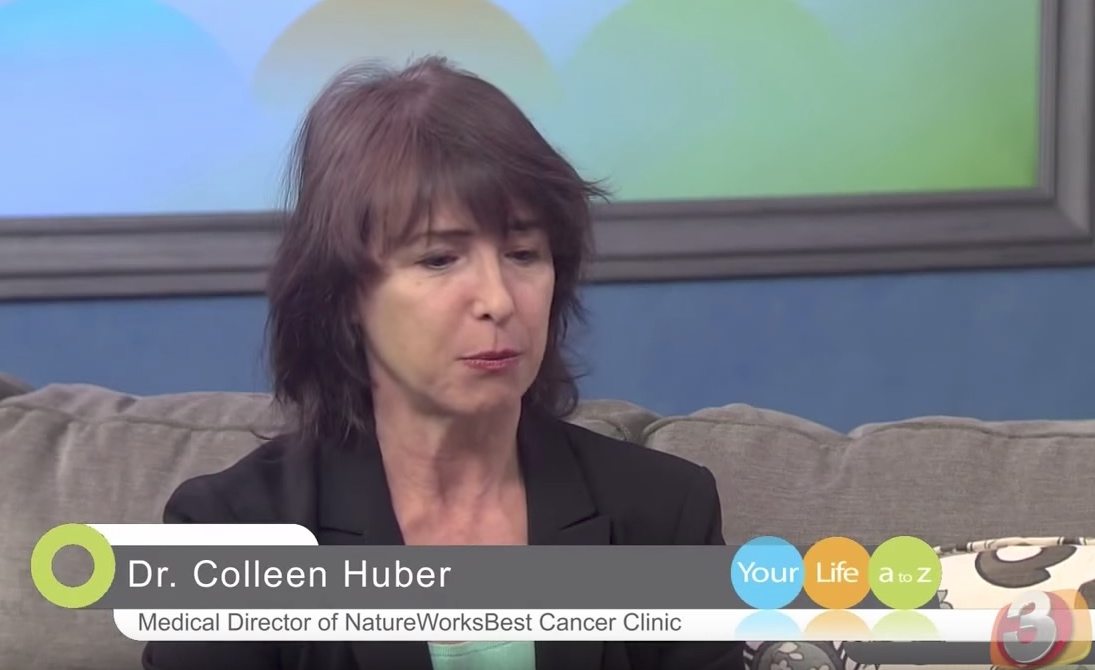
Cancer quack Colleen Huber sues Britt Hermes over criticism
Naturopathic cancer quack Colleen Huber is attempting to silence criticism of her practices by suing Britt Hermes. Help Britt fight back with a donation to help defray legal expenses.

Another “Chronic Lyme” VIP disciplined by NY medical authorities: Bernard Raxlen
Another "Lyme literate" NY physician is on probation and under orders to clean up his act. Will other physicians treating "chronic Lyme" take note?

Reiki: Fraudulent Misrepresentation – Revisited
Factual misrepresentations about manipulating "energy" in a patient's body and its positive effects on health are integral to reiki. They can also be the basis of an action for fraudulent misrepresentation.

Naturopathic Cancer Quackery
Naturopaths treat cancer with an array of implausible concoctions that are not based on clinical science, and then defend themselves with cease and desist letters.

No, a Vaccine Court ruling does not show that vaccines cause SIDS
There was a rumbling in the antivaccine underground over the weekend about a recent ruling by the Vaccine Court compensating parents of a child who died of sudden infant death syndrome (SIDS). In a confused and scientifically highly flawed decision, the Special Master Thomas Gowen didn't rule that vaccines cause SIDS, but did rule that they contributed to SIDS in this one...

Abraham Cherrix is alive and well because of science-based medicine
Although I haven't discussed it here in depth, the case of Abraham Cherrix is an instructive example. Eleven years ago, he and his parents chose quackery over science-based medicine to treat his cancer. He's alive now because he finally realized the error of his decision and underwent chemotherapy and a bone marrow transplant.

Amish Farmer Jailed for Selling Snakeoil
An Amish farmer is convicted of selling a caustic poison as patent medicine (and of witness tampering) and yet is defended by "alternative medicine" proponents who apparently want the freedom to be defrauded and harmed.

Medical Neglect of Children
Medical neglect caused horrific suffering for these children, ending in death or permanent impairment. Their parents failed them, but so did society.
Legislative Alchemy 2017: Naturopaths push licensing and practice expansion while in damage control over IV turmeric death
Naturopaths are in damage control mode over the death of a naturopathic patient due to turmeric infusion, even as they lobby state legislatures for licensing and practice expansion.