Category: Pharmaceuticals
Do anti-inflammatory drugs effectively treat spinal pain?
While anti-inflammatory drugs are commonly used to treat back pain, a new review suggests that they may not provide meaningful benefits to most people.
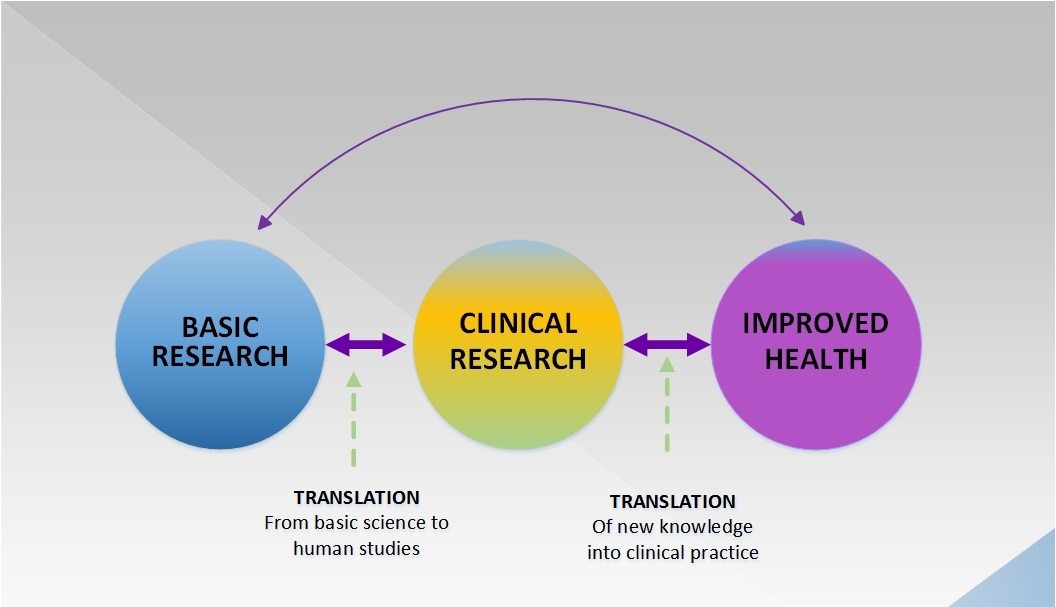
Donald Trump versus the FDA: Is the standard of evidence for drug approval actually too low rather than too high?
All of the candidates being considered by President Trump for FDA Commissioner believe that the FDA is too strict in its standards for approving new drugs. In a commentary in Nature last week, two bioethicists argued that, at least in terms of preclinical data, the standard of evidence is actually too low. Which is correct?
How accurately do physicians estimate risk and benefit?
A new study suggests that physicians tend to overestimate the benefits of treatments, tests, and screening tests, while also underestimating harms.

Donald Trump and Peter Thiel vs. the FDA: Be afraid. Be very afraid.
Donald Trump's three most likely picks for FDA Commissioner all favor loosening drug approval standards. Two are cronies of Peter Thiel, of which one believes that the FDA shouldn't require evidence of efficacy, only safety, and the other believes that a "Yelp for drugs" would do a better job than the FDA. The third candidate is a bona fide, honest-to-goodness pharma shill....

Drug therapy is still sending too many people to the emergency department
Prescription drugs continue to send thousands to the emergency room every year. Many of these adverse drug events are predictable and avoidable.

Medical science policy in the U.S. under Donald Trump
The election of Donald Trump was unexpected. Given Trump's history of antivaccine beliefs and conspiracy theories, coupled with a fervor for deregulation (a fervor shared by the Republican Congress), it is reasonable to fear what will happen to medical science policy during the next four years.

R&D and the High Cost of Drugs
Until a year ago very few people had ever heard of Martin Shkreli. In 2015 the then-32-year-old CEO of Turing Pharmaceuticals LLC became the poster boy for Big Pharma eXXXcesses when Turing acquired rights to Daraprim, an antiparasitic drug used widely to treat toxoplasmosis. The acquisition itself wasn’t particularly controversial. Raising the price of Daraprim from $13.50 per pill to $750 per...
Do pill organizers help or hurt?
In order for medication to work, getting a prescription filled isn’t enough. You have to actually take the medication. And that’s where you (the patient) come in. Estimates vary based on the population and the medication, but a reasonable assumption is that 50% of people given a prescription don’t take their medication as prescribed. In pharmacy terminology we usually call this medication...
Whither the randomized controlled clinical trial?
With the rise of precision medicine and genomics, the conventional randomized clinical trial appears more and more outdated. Fortunately, clinical trials are evolving, but will it be enough to incorporate the numerous advances in "-omic" medicine in a rigorous scientific manner to benefit patients?

Is there a reproducibility “crisis” in biomedical science? No, but there is a reproducibility problem
Reproducibility is the key to scientific advancement. It has been claimed that we suffer from a "reproducibility crisis," but in reality it is a chronic problem in reproducibility. Here we will look at the scope of the problem and strategies to address it.

