Category: Pharmaceuticals
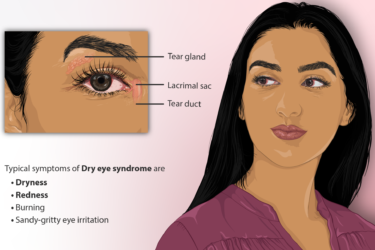
Eye Drops for Dry Eyes
I could have chosen a prescription eye drop for my dry eyes. I decided not to. Here's why.
A New Medication to Combat Obesity
New study in The New England Journal of Medicine finds impressive evidence that weekly semaglutide injections produce clinically significant weight loss as well as many other benefits, approaching the improvements seen with weight loss surgery. Not a definitive answer to obesity, but a very encouraging step in the right direction. Science works.
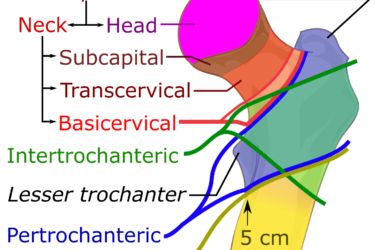
Evenity for Osteoporosis
Hip and wrist fractures are a common result of osteoporosis. A new drug, Evenity, reduces the risk of vertebral fractures, but it doesn't significantly reduce the risk of non-vertebral fractures. Other drugs do.

Statin Side Effects Revisited
Patients on statins frequently report muscle pain and other side effects, but controlled studies have shown side effects are not more frequent than with placebo. Why this discrepancy? A new study sheds some light.
Deliberately Spoiled Vaccines: Conspiracy thinking and health professionals
A Wisconsin pharmacist is facing charges after deliberately tampering with a batch of COVID-19 vaccines, demonstrating that a health professional's education is no vaccine against conspiracy beliefs.

Salonpas
Salonpas is an over-the-counter topical NSAID used to treat pain. It's probably safe and might be worth trying for minor pain, but the effect is small and the advertising is more hype than substance.

Progeria
The first drug to treat Hutchinson-Gilford Progeria, a rare and uniformly fatal rapid aging disease, has been approved by the FDA. It can prolong the life of these children by 2.5 years, but it is very expensive.
Government watchdog warns about paid physician speeches touting drugs and medical devices
Paid speeches at lavish events touting drugs and medical devices risk charges of anti-kickback law violations, warns a recent government Special Fraud Alert. Will companies and doctors take heed this time?
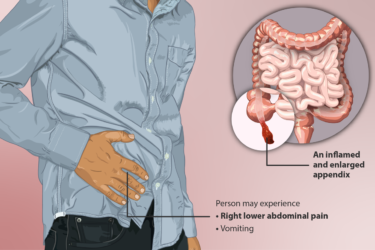
Appendicitis: Surgical vs. Medical Treatment
Surgery or antibiotics for appendicitis? This new study can help with the decision.
When Doctors Refuse to Believe Evidence
Paul Offit's new book covers the evidence for many surgeries, medications, and screening tests that have been proven ineffective and harmful yet are still being used by doctors who refuse to follow the science.

