Category: Pharmaceuticals

Jumping the Gun: Antibody Drugs for Covid-19
Why did the US government promise to buy a product that had failed testing?

Importing drugs from Canada won’t lower drug prices for Americans
The Trump administration has approved the bulk importation of prescription drugs from Canada. But Canada isn't on board with this plan, and it's not going to reduce drug prices for Americans.
In the age of the COVID-19 pandemic, can we trust the CDC and FDA any more?
Since the COVID-19 pandemic reached the US, increasing concern has been expressed about the politicization of the CDC and FDA due to pressure from the Trump administration to downplay the severity of the pandemic and push out treatments and a vaccine as fast as possible, potentially at the expense of safety. This has led me to a disturbing question: Can I trust...

FDA warns companies selling illegal hangover remedies
The FDA recently warned seven companies not to claim that their dietary supplements can prevent, treat, or cure a hangover, because only FDA-approved drugs can make such claims. The agency also warned that NAC, a popular supplement ingredient, cannot legally be used in dietary supplements.

The TikTok “Benadryl Challenge” is demonstrating how toxic Benadryl can be
Even without the risk of overdose, there are good reasons to avoid Benadryl entirely.
Dexamethasone and Hydroxychloroquine: Why Randomized Controlled Trials Matter
What does the best evidence tell us about hydroxychloroquine and dexamethasone?
Shooting the Messenger: Activists Persecute Scientists Whose Findings They Don’t Like
Alice Dreger's book recounts many instances of shooting the messenger, when scientists were persecuted for research findings that activists found objectionable. Social justice matters, but it should rely on science and reality, not ideology.

Expert review warns against compounded bioidentical hormone therapies
A National Academies report finds widely-marketed compounded hormone replacement therapies lack evidence of safety and effectiveness, and recommends restriction of their use.

Probiotics, revisited
New guidelines do not recommend probiotics for most gastrointestinal conditions.
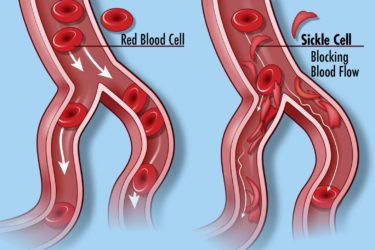
New Drugs for Sickle Cell Disease: Small Benefit, Large Price
The FDA has approved two new drugs to treat sickle cell disease. They don’t do much, and they are prohibitively expensive.

