Review
One of the more bizarre and unpleasant “CAM” claims, but one taken very seriously at the NIH, at Columbia University, and on Capitol Hill, is the cancer “detoxification” regimen advocated by Dr. Nicholas Gonzalez:
Patients receive pancreatic enzymes orally every 4 hours and at meals daily on days 1-16, followed by 5 days of rest. Patients receive magnesium citrate and Papaya Plus with the pancreatic enzymes. Additionally, patients receive nutritional supplementation with vitamins, minerals, trace elements, and animal glandular products 4 times per day on days 1-16, followed by 5 days of rest. Courses repeat every 21 days until death despite relapse. Patients consume a moderate vegetarian metabolizer diet during the course of therapy, which excludes red meat, poultry, and white sugar. Coffee enemas are performed twice a day, along with skin brushing daily, skin cleansing once a week with castor oil during the first 6 months of therapy, and a salt and soda bath each week. Patients also undergo a complete liver flush and a clean sweep and purge on a rotating basis each month during the 5 days of rest.
Veteran SBM readers will recall that in the spring of 2008 I posted a series of essays* about this regimen and about the trial that compared it to standard treatment for subjects with cancer of the pancreas. The NIH had funded the trial, to be conducted under the auspices of Columbia, after arm-twisting by Rep. Dan Burton [R-IN], a powerful champion of quackery, and much to the delight of the “Harkinites.”
In the fall of 2008 I posted an addendum based on a little-known determination letter that the Office for Human Research Protections (OHRP) had sent to Columbia during the previous June. The letter revealed that the trial had been terminated in October, 2005, due to “pre-determined stopping criteria.” This demonstrated that Gonzalez’s regimen must have been found to be substantially worse than the current standard of care for cancer of the pancreas, as ineffective as that standard may be. I urge readers who require a review or an introduction to the topic to read that posting, which also considered why no formal report of the trial had yet been made available.
Now, finally, the formal report has been published online by the Journal of Clinical Oncology (JCO):
Pancreatic Proteolytic Enzyme Therapy Compared With Gemcitabine-Based Chemotherapy for the Treatment of Pancreatic Cancer
John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, and Victor R. Grann
JCO Early Release, published online ahead of print Aug. 17, 2009. Journal of Clinical Oncology, 10.1200/JCO.2009.22.8429
The results of the Gonzalez trial
The abstract can be read here. First, the punch line:
Conclusion: Among patients who have pancreatic cancer, those who chose gemcitabine-based chemotherapy survived more than three times as long (14.0 v 4.3 months) and had better quality of life than those who chose proteolytic enzyme [Gonzalez’s] treatment.
According to the article proper,
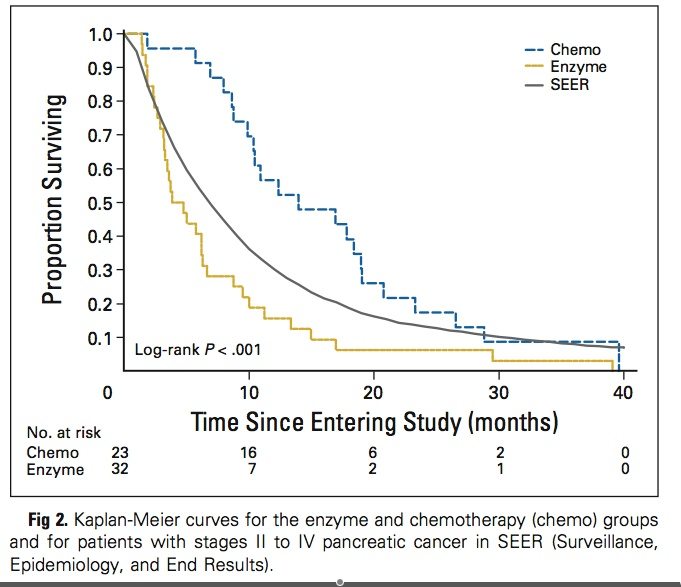
Twelve months after enrollment, 56% of chemotherapy-group patients were alive; 16% of the enzyme-group patients were alive. The longest survivors were one chemotherapy-group patient who died at 39.5 months and one chemotherapy-group patient who was censored at 37.5 months (ie, the closing date of the data analysis) and, at the time of manuscript submission, was still alive at 40 months.
The Gonzalez regimen was not only much worse than standard treatment in this study; it was also substantially worse than the experience of more than 20,000 comparable patients in the US between 1988 and 2001, as reported by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. This is illustrated in a self-explanatory figure from the JCO paper:
The Columbia trial also looked at ‘quality of life’:
In this study, patients in the enzyme-treatment and chemotherapy groups had similar scores in quality of life at enrollment, but the enzyme group fared much worse than the gemcitabine group in the subsequent year…Pain levels appeared to diverge during the initial 6 months of the study and were more severe among the enzyme group.
Discussion
This is a slam-dunk condemnation of the Gonzalez regimen. For more than 20 years, Gonzalez has claimed near-miraculous survival times—measured in years-to-decades, rather than in months—for patients with life-threatening cancer, including pancreatic cancer. His “case series,” upon which the NIH’s decision to fund a large trial was purportedly based, had included 11 patients. According to the National Cancer Institute (NCI):
The investigators reported a median survival time of 17 months and a mean survival time of 25.2 months for these patients. Nine patients (82%) survived 1 year, five patients (45%) survived 2 years, and four patients (36%) survived 3 years. At the time the study was reported, two patients were alive: one who had survived 3 years, and one who had survived 4 years. The researchers concluded that the 1-year and 2-year survival percentages for this group of patients were superior to those observed for other U.S. patients diagnosed with adenocarcinoma of the pancreas (1-year survival, all stages = 25%; 2-year survival, all stages = 10%).
I have previously explained (here and here), as has Dr. Peter Moran (here), why that case series was unreliable and was not a worthy basis for conducting a larger trial.
Dr. Gonzalez has also claimed that patients who use coffee enemas “report an increased sense of well being.” He reportedly told at least one subject in the Columbia trial
that pain might be an indication that the tumors were being dissolved, and that he could expect weight loss as he was detoxifying his body.
The JCO report soundly refutes Gonzalez’s claims. The treatment does not prolong the lives of patients with cancer of the pancreas; it substantially shortens their lives. It does not increase patients’ sense of well being; rather, it makes their quality of life “much worse.” The fate of the typical subject in the “enzyme” group appears to have been similar to that of a young man whose tragic story was told by his friend, Susan Gurney, and recounted here.
Is this the last word on the Gonzalez regimen? Are there weaknesses in the study that might save it, at least for now? I raise this question for completeness’ sake only, for in my opinion there was never a justification for performing the trial. Nevertheless, I’ll mention the only obvious methodologic flaw: As previously discussed, the trial was not randomized because at its outset too few prospective subjects were willing to take a chance on not being in the “Gonzalez” group. Thus allocation to that group was chosen by the subjects themselves. They were compared to a cohort of patients who chose standard treatment. Although not randomly allocated, the subjects in the two groups were reported to be well matched:
Fifty-five patients, 23 on the control arm and 32 on the experimental arm, enrolled on the study and were available for analysis… The patients in both the control and experimental arms were carefully enrolled according to identical entry criteria. There were no statistically significant differences at the time of enrollment in age, sex, weight, ECOG performance status, stage of disease, pathology, quality of life, or CA19-9. Bilirubin and albumen were significantly higher in the chemotherapy group, but all values were clinically within normal limits and met eligibility criteria.
All but two subjects in each group, moreover, were judged Grade 0 (“Fully active, able to carry on all pre-disease performance without restriction”) or Grade 1 (“Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work”) by the Eastern Cooperative Oncology Group (ECOG) Performance Status assessment. This is important because, as I reported last year, someone (probably Gonzalez or a surrogate) had complained to the OHRP that several subjects who would not be capable of adhering to the “enzyme” regimen had been enrolled in the trial (see points 5-7 in the OHRP letter).
The lack of randomization (and blinding), then, seems insufficient to cast doubt on such a definitive outcome. It is likely, moreover, that any bias resulting from self-selection of subjects to the Gonzalez group would have skewed the results in favor of that regimen—the opposite of what was ultimately found. Gonzalez himself seemed to realize this back in 2000, when the trial had barely begun:
DR. GONZALEZ: Interesting question. I didn’t have time to go into the details of how it was going. Initially, it was set up as a randomized trial about a year ago, and Jeff and I kind of struggled with that and I always knew there would be problems.
And—–going back to this gentleman’s question, I’m the first person on Earth to say that the belief system of the patient is really important in how they do. Randomized trial patients have no choice and the study was set up when my therapy was being compared to the best available chemotherapy for inoperable pancreatic adenocarcinoma.
Well, patients know in this day and age chemotherapy is a death sentence for inoperable pancreatic adenocarcinoma and our first study had already been published.
Over a period of several months, Columbia got 200 phone calls. A hundred and ninety-seven patients, who would have been perhaps appropriate for the study, refused to enter unless they could be guaranteed my arm of the study.
Now a randomized study is considered the gold standard and I felt we had to at least try. Dr. Klausner thought we had to try. Dr. White thought we had to try. After a year of trying that we all realized it wasn’t going to work that way, so we set it up as a case control where patients have choice. If they want chemo, they get chemo; if they want me, they get me.
Hopefully, their belief in chemo will be as strong as the patients’ belief in my therapy. We’re going to try and match them evenly.
Now in terms of technical methodology it’s maybe a little less rigorous than a randomized study, but it’s raised all kinds of issues about how you test an alternative therapy where belief is important and a patient who doesn’t care whether they get chemo or my therapy for pancreatic cancer is going to be a very unusual patient.
Patients who come to me usually believe in this. They would seek alternatives. By definition, they’re seeking something different.
At the same conference, Gonzalez opined that in order to validate his regimen, the trial would eventually need to demonstrate outcomes virtually the inverse of what have now been reported:
DR. GONZALEZ: It’s set up as a survival study. We’re looking at survival.
SPEAKER: Do you have an idea of what you’re looking for?
DR. GONZALEZ: Well, Jeff and I were just talking a couple weeks ago. You know, to get any kind of data that would be beyond criticism is—-always be criticism, but at least three times.
You would want in the successful group to be three times — the median to be three times out from the lesser successful groups.
So, for example, if the average survival with chemo, which we suspect will be 5 months, you would want my therapy to be at least — the median survival to be at least 15, 16, 17 months, as it was in the pilot study.
We’re looking for a median survival three times out from the chemo group to be significant.
To save you the trouble of scrolling upward, let me repeat the JCO report’s conclusion:
Among patients who have pancreatic cancer, those who chose gemcitabine-based chemotherapy survived more than three times as long (14.0 v 4.3 months) and had better quality of life than those who chose proteolytic enzyme treatment.
There is More to This Story
I am happy that the results of the Gonzalez trial, such as they are, have finally been made public. Nevertheless, the report is full of troubling information, both stated and unstated. There is substantial evidence of ethical breaches, including some that might elude readers who are not familiar with the topic. A compelling argument can even be made that the JCO should not have published the report—as paradoxical as that sounds. I’ve previously discussed several of these issues (also here), but the JCO report demands that they be revisited. I’ll do that next week.
* The “Gonzalez Regimen” Series:
1. The Ethics of “CAM” Trials: Gonzo (Part I)
2. The Ethics of “CAM” Trials: Gonzo (Part II)
3. The Ethics of “CAM” Trials: Gonzo (Part III)
4. The Ethics of “CAM” Trials: Gonzo (Part IV)
5. The Ethics of “CAM” Trials: Gonzo (Part V)
6. The Ethics of “CAM” Trials: Gonzo (Part VI)
7. The “Gonzalez Trial” for Pancreatic Cancer: Outcome Revealed
8. “Gonzalez Regimen” for Cancer of the Pancreas: Even Worse than We Thought (Part I: Results)
9. “Gonzalez Regimen” for Cancer of the Pancreas: Even Worse than We Thought (Part II: Loose Ends)
REFERENCE:
John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, & Victor R. Grann (2009). Pancreatic Proteolytic Enzyme Therapy Compared With Gemcitabine-Based Chemotherapy for the Treatment of Pancreatic Cancer Journal of Clinical Oncology : 10.1200/JCO.2009.22.8429 (E-pub ahead of print)
