Category: Clinical Trials
COVID-19 vaccine-caused “turbo cancer” nonsense just keeps getting more turbocharged
No matter how implausible it is or how weak the evidence for it is, the myth that COVID vaccines cause "turbo cancer" just won't die. Quite the contrary, alas. Antivaxxers are—dare I say?—turbocharging it with bad science.

Fenbendazole is fast becoming the laetrile of the 2020s
Antivaxxers who "repurposed" deworming drugs like ivermectin and fenbendazole are peddling cancer "miracle cure" testimonials that remind me of laetrile and Stanislaw Burzynski. Truly, everything old is new again.

Adios Aduhelm
The controversial and never-proven-effective drug to treat Alzheimer's disease, aducanumab (Aduhelm) has been discontinued.
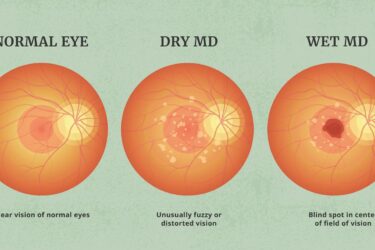
Pegcetacoplan (Syfovre™) for macular degeneration: an update
SBM's guest contributor and ophthalmologist, Dr. David Weinberg, provides an update on the phase 3 trials of pegcetacoplan for macular degeneration. The results are still disappointing.
2023: The year that the evidence-based medicine (EBM) paradigm was weaponized against vaccines and public health
Evidence-based medicine (EBM) has been a very useful paradigm for assessing evidence in medicine. However, like any other framework, it can be misused, particularly when fundamentalist EBM methodolatry leads to its inappropriate application to questions for which it is ill-suited, a misuse that has been weaponized against public health during the pandemic.

Make Acupuncture Great Again
Calling a losing study a win. Making acupuncture great again.
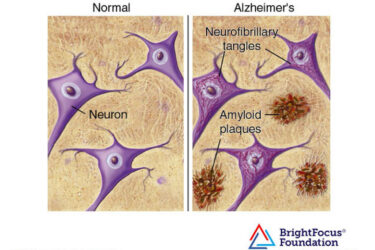
Yet Another Alzheimer’s Drug
A third drug either FDA approved or showing clinical benefit for Alzheimer's disease confirms that the world of AD treatment has changed forever.

The effects of vitamin D supplementation on major cardiac events
A large randomized controlled trial of vitamin D supplementation generates good data to show there is likely no benefit.

RFK Jr. resurrects an old antivax half-truth about “saline placebos” in randomized controlled trials of vaccines
Robert F. Kennedy, Jr. has resurrected the antivax claim that the childhood vaccine schedule has never been tested in randomized controlled trials (RCTs) with a saline placebo controls (and therefore the vaccine schedule is unsafe). This is an old and deceptive antivax half-truth that ignores both what constitutes a scientifically valid placebo and the ethical requirements for RCTs.


Courage
If I were King of the Vaccines. Some thoughts on influenza vaccine, hospitalization, death and courage.