Category: Medical Ethics
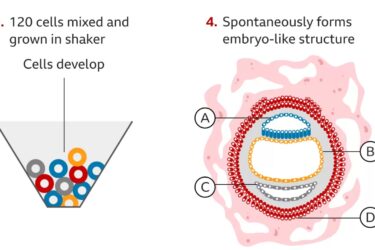
Complete Human Embryo Model Made From Stem Cells
Researchers create an embryo model from human embryonic stem cells.

Examining COVID-19 misinformation propagated by US physicians
A new paper documents COVID-19 medical misinformation shared by US physicians on social media

COVID-19 vaccines and the Nuremberg Code
Antivaxxers love to claim that vaccine mandates (especially COVID-19 vaccine mandates) violate the Nuremberg Code and call for Nuremberg-style tribunals to hold public health and vaccine advocates "accountable". As usual, they have no idea what they are talking about. This is also not a new antivax narrative, although what is unprecedented is that what was once fringe even among antivaxxers is now...
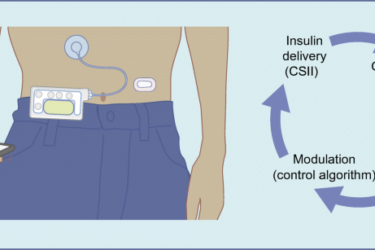
DIY Diabetes Treatment
Can DIY medicine work?

About those “19 Errors,” Part Two
A critical and evidence-based response to the alleged errors journalist Jesse Singal found in my guest post reviewing Abigail Shrier's book on trans youth. The second of two parts.

About those “19 errors,” part one
Journalist Jesse Singal took issue with Dr. Rose Lovell and Dr. AJ Eckert's guest posts about Abigail Shrier's book on transgender teens, Dr. Harriet Hall's review of the book, and the medical care of these teens, claiming that the posts contained "19 errors." In this post, Dr. Lovell responds to her share of these "19 errors," most of which are not errors.

Abigail Shrier’s Irreversible Damage: A Wealth of Irreversible Misinformation
A critical, science-based analysis of Abigail Shrier's book Irreversible Damage.
Government watchdog warns about paid physician speeches touting drugs and medical devices
Paid speeches at lavish events touting drugs and medical devices risk charges of anti-kickback law violations, warns a recent government Special Fraud Alert. Will companies and doctors take heed this time?

Would you pay $1 million to enroll in a phase 1 clinical trial of an “anti-aging” gene therapy?
Libella Gene Therapeutics, LLC made the news last week for announcing a "pay-to-play" trial of its telomerase-based anti-aging gene therapy. What was shocking about the announcement was not that it was a "pay-to-play" trial, given that such trials have become all too common, but rather the price of enrollment: $1 million. Worse, the trial is being conducted in Colombia; the therapy doesn't...
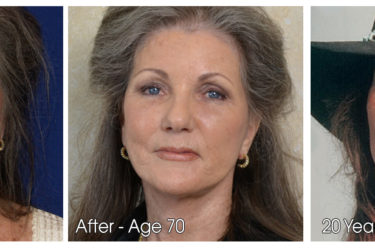
Nurse Practitioner Pushes Dubious Aesthetic Treatments
Nurse practitioner aggressively advertises a plethora of aesthetic treatments, some of which are dubious. It's legal, but is it ethical?

