Category: Pharmaceuticals

SARMs Harms
Selective androgen receptor (SARMs) are sports supplements marketed to teens via social media influencers.

AI for prescription drug information: Not yet useful for health care – but it’s coming
ChatGPT may not replace a health care professional's assessment yet, but its capabilities are growing.

Adios Aduhelm
The controversial and never-proven-effective drug to treat Alzheimer's disease, aducanumab (Aduhelm) has been discontinued.
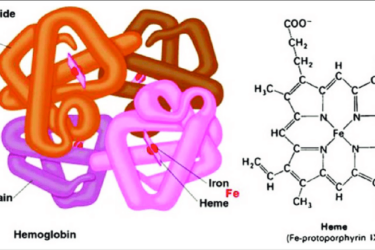
First CRISPR Treatment Approval
The first CRISPR-based therapy approval is a milestone worthy of noting.

Decongestant doesn’t work, concludes FDA advisory committee
An FDA advisory committee has concluded that phenylephrine, a popular decongestant in cough and cold remedies, is ineffective.

The (Cold) Drugs Don’t Work
The FDA continues to permit the inclusion of an ineffective ingredient in hundreds of cough and cold products.

How the “Don’t take this medication with grapefruit juice” warning originated
How a chance discovery by one scientist improved the safety of consumers worldwide.
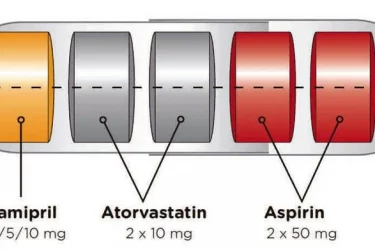
The Polypill Comes of Age
The polypill is effective for secondary prevention of cardiovascular disease. By combining drugs in a single pill, it improves convenience and compliance.

PreP for HIV/AIDS
Two pills and an injection are FDA-approved to prevent HIV infection. Not enough patients and providers know about them.

Zinc for the prevention or treatment of respiratory tract infections: A new systematic review
A new systematic review examines the evidence for zinc to prevent or treat respiratory tract infections.

