Category: Pharmaceuticals
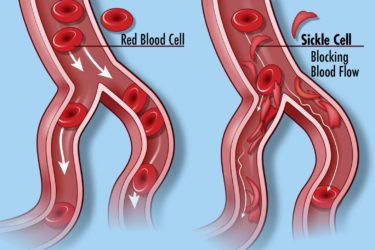
New Drugs for Sickle Cell Disease: Small Benefit, Large Price
The FDA has approved two new drugs to treat sickle cell disease. They don’t do much, and they are prohibitively expensive.

No, Everything You Thought You Knew About Disease Is Not Wrong
The authors of this book are not doctors or scientists, but they try to convince readers that science-based medicine gets it all wrong, that germs don't cause disease, and that drugs and vaccines can't possibly work. No, the book gets it all wrong.
COVID-19: Out-of-control science and bypassing science-based medicine
During the COVID-19 pandemic, there hasn't just been a pandemic of coronavirus-caused disease. There's also a pandemic of misinformation and bad science. It turns out that doctors today are just as prone as doctors 100 years ago during the 1918-19 influenza pandemic to bypass science-based medicine in their desperation to treat patients.
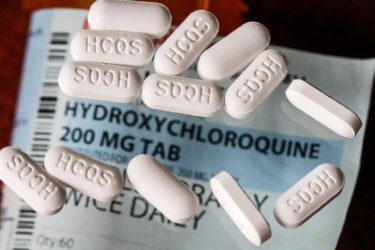
Hydroxychloroquine and the price of abandoning of science- and evidence-based medicine
Based on anecdotal evidence early in the pandemic and then-unreported clinical trials, followed by hype and bad studies by French "brave maverick doctor" Didier Raoult, antimalarial drugs hydroxychloroquine and chloroquine became the de facto standard of care for COVID-19, despite no rigorous evidence that they worked. A steady drip-drip-drip of negative studies has led doctors and health authorities to rethink and backtrack,...
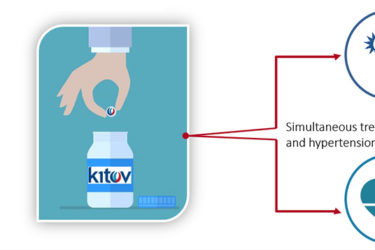
Fixed Dose Combination Drugs: Consensi Is a Bad Example
Consensi combines two drugs for high blood pressure and osteoarthritis. That doesn't make sense, and it costs $12,000 a year more than taking the individual components.
More drug shortages due to COVID-19
COVID-19 is worsening a long-standing problem: Drug shortages

TCM for COVID-19
Despite the many claims, there is no real evidence that traditional Chinese medicine (TCM) is effective for prevention or treatment of COVID-19
Drug shortages are worsening, and there are no simple solutions
Drug shortages, which worsen medical care and patient outcomes, are becoming more and more common. A new Task Force report from the FDA offers a potential way forward.

There’s No Vaccine for HIV/AIDS, But There’s Truvada
Science has made great strides in understanding, treating, and preventing HIV/AIDS. We can hope for an AIDS vaccine, but meanwhile there is a pill that can markedly reduce the risk of becoming infected.

FDA proposes ban on curcumin and other naturopathic favorites in compounded drugs
Based on a thorough review of the evidence by experts, the FDA is proposing a ban on using curcumin, cesium chloride and other naturopathic favorites in compounded drugs.

