Category: Politics and Regulation

SARMs Harms
Selective androgen receptor (SARMs) are sports supplements marketed to teens via social media influencers.
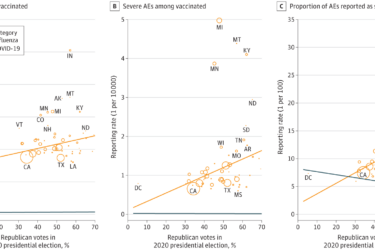
Dr. Vinay Prasad vs. a VAERS study finding more reports of vaccine injury in red states
Dr. Vinay Prasad attacks an epidemiological study published in JAMA Open Network reporting that people in red states are more likely to report vaccine injuries, claiming that a more rigorous study would "not be difficult," when he knows that it would be very difficult.

Pesticide in Oat Products – Should You Worry?
You know the rule about headlines - if there is a question in a headline the answer is almost always "no". This article is no exception.

Adios Aduhelm
The controversial and never-proven-effective drug to treat Alzheimer's disease, aducanumab (Aduhelm) has been discontinued.
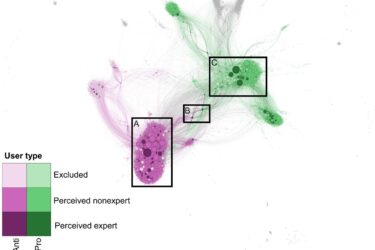
Yet more evidence that we physicians need to clean up our act
A recent study found that physicians and scientists who are perceived as "experts" are prevalent within the antivax community and more influential because of their status as physicians and scientists. Why do physicians continue to tolerate antivax quacks within our ranks?

COVID-19 antivax quacks are now “repurposing” ivermectin for cancer
A year ago, I noticed that COVID-19 quacks were touting the "repurposing" of ivermectin to treat cancer. Now, familiar COVID-19 antivaxxers—cough, cough, FLCCC—have turbocharged this quackery.

FDA: Don’t use homeopathic eye drops
There are no homeopathic eye drops approved by the FDA.
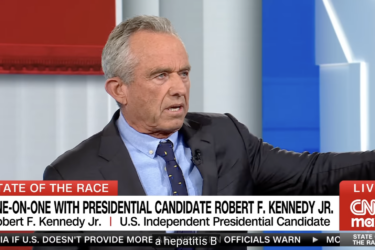
RFK Jr. and his “I’m not anti-vaccine” rejoinder to being confronted with his past antivax statements: A primer
On Friday, CNN host Kasie Hunt interviewed antivax presidential candidate Robert F. Kennedy, Jr. Although she did better than most journalists confronting him for his past antivax statements in that she played a clip of one of his antivax statements, she clearly hadn't anticipated his response, which should have been very predictable given that he's been using it for at least 15...

When a “gender critical” is a runner-up for the Maddox Prize for standing up for science…
Helen Joyce, a virulently anti-trans "gender critical" campaigner, was recently shortlisted for the Maddox Prize, which purports to recognize people who "who stand up for science and evidence, advancing public discussion around difficult topics despite challenges or hostility," even though she promotes an agenda that denies science and demonizes trans people. How could this have happened?


