Category: Politics and Regulation
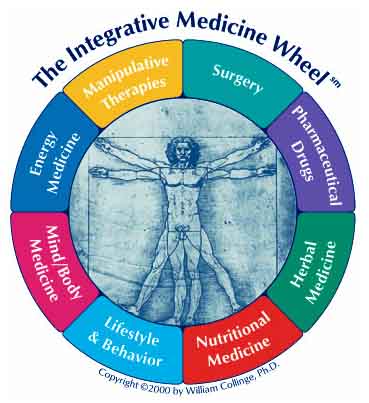
State Medical Boards should not recognize board certification in “Integrative Medicine”
Integrative medicine is not a real specialty in medicine. Let's not treat it as though it were.

FTC Homeopathy Win
I love to see a regulatory agency actually do its job. Especially within medicine, where it is most important, the lack of political will seems to get in the way of properly regulating health care products and services in the way that most consumers assume they are regulated. Homeopathy is perhaps the best example. Homeopathy is pure unadulterated pseudoscience and witchcraft. There...

Medical science policy in the U.S. under Donald Trump
The election of Donald Trump was unexpected. Given Trump's history of antivaccine beliefs and conspiracy theories, coupled with a fervor for deregulation (a fervor shared by the Republican Congress), it is reasonable to fear what will happen to medical science policy during the next four years.

“Donald Trump’s presidential election win stuns scientists”
Scientists in the U.S. and from around the world are weighing in on Donald Trump’s election as the next president of the most powerful country on earth: Trump will be the first anti-science president we have ever had . . . The consequences are going to be very, very severe. Michael Lubell, director of public affairs for the American Physical Society in...

Cancer quackery from Germany to Australia
Sadly, cancer quackery is a worldwide phenomenon. Here, we examine its reach from Germany to Australia.

California legislature should repeal naturopathic licensing
On January 1, 2018, the California Naturopathic Doctors Act will be automatically repealed unless the California Legislature deletes or extends that date during the 2017 legislative session, which convenes on December 5, 2016. In addition, according to California law, the Naturopathic Medicine Committee of the Osteopathic Medical Board of California, which regulates naturopathic doctors (NDs), is subject to review by “appropriate policy...
Natural Health Products: Loosely regulated, little evidence of benefit, and an industry intent on preserving the status quo
This week’s post will revisit a topic I recently covered, but it’s time-sensitive and needs your input. Health Canada, the Canadian equivalent to the US Food and Drugs Administration, is considering revisions to the way in which it regulates dietary supplements, which are called “natural health products” in Canada. It is rare that a regulator acknowledges that a regulatory system isn’t working,...

Is there a distinct standard of care for “integrative” physicians? The Woliner case
We at SBM argue that there should be a single, science-based standard of care in medicine. Unfortunately, with the rise of "complementary and alternative medicine" (CAM) also called "integrative medicine," there is a separate standard emerging that allows CAM practitioners to get away with using unproven and disproven treatments. The case of Dr. Kenneth Woliner illustrates this problem.
In which we are accused of “polarization-based medicine”
A little over a month ago, I wrote about how proponents of “complementary and alternative medicine” (CAM), now more frequently called “integrative medicine,” go to great lengths to claim nonpharmacological treatments for, well, just about anything as somehow being CAM or “integrative.” The example I used was a systematic review article published by several of the bigwigs at that government font of...

Personal Care Products Safety Act: Facelift for FDA Regulation or Lipstick on a Pig?
The U.S. cosmetics industry, the largest in the world, is expected to reach $62 billion in revenues in 2016. Yet, despite the fact that its products are regularly applied to, and absorbed by, the body’s largest organ (the skin) and even ingested in small amounts, the cosmetics industry is largely self-regulating. There are over 57,000 different chemicals used in cosmetics. According to...

