
Ask your pharmacist if nothing is right for you. (HT @leachkathleen)
On April 21 and 22, the FDA held a public hearing:
to obtain information and comments from stakeholders about the current use of human drug and biological products labeled as homeopathic, as well as the Agency’s regulatory framework for such products. . . . FDA is seeking participants for the public hearing and written comments from all interested parties, including, but not limited to, consumers, patients, caregivers, health care professionals, patient groups, and industry.
The FTC recently announced that it, too, is wading into the homeopathic waters. The FTC, which regulates advertising of homeopathic products, will hold a public workshop on September 21 in Washington, DC, “to examine advertising for over-the-counter (OTC) homeopathic products.” Like the FDA, it will also accept public comments online.
All of this regulatory buzz caused the FDA Law Blog to take notice. (The blog is hosted by a law firm specializing in food and drug regulation law.) A post titled “Will FTC Kill Homeopathic Products – or Will FDA?” gave this assessment:
Bottom line, if the FTC holds homeopathic products to the same scientific standards that are applied to claims for other OTC products like dietary supplements, as the FTC appears inclined to do . . . few if any homeopathic products will pass the test.
Actually, the FTC should hold homeopathic products to the same scientific standards as are applied to claims for OTC drugs, not dietary supplements, for that is how Congress classifies them. But we won’t be picky if the result is as FDA Law Blog suggests. Nor do we care who the killer is, as long as it gets done.
The homeopathic industry responded in spades to the FDA’s invitation (as I imagine it will to the FTC’s) trotting out not only its own representatives, but also sympathizers from the integrative and alternative medicine communities and consumers. These greatly outnumbered the voices of reason and science, but the industry and its acolytes were impressive in number only. The vast majority of the 7,000 online comments have also been pro-homeopathy, but that appears to have backfired, for reasons we’ll get to in a minute. (There’s still time to comment – the deadline was just extended until August 21.)
But before we get to the homeopathic dog-and-pony show, a brief background: Back in 1938, Congress passed the Food, Drug and Cosmetic Act, which required drug manufacturers to demonstrate safety before their products could be marketed. In 1962, the Act was amended to require a demonstration of efficacy as well. Although Congress specifically included homeopathic drugs, the FDA has never required the homeopathic industry to demonstrate safety or efficacy of it products prior to putting them on the market. The FDA does have homeopathic-friendly labeling requirements and the industry is required to employ good manufacturing practices. The FDA can go after a homeopathic product post-market if it presents a safety issue or is not properly labeled, but that’s it.
Interestingly, the very situation that prompted Congress to pass drug regulation laws at the beginning of the 20th century perfectly describes the homeopathic industry today. As FDA historian John Swann, PhD, recounts, back in the day:
Lee Barlett, a former shirt salesman from Pittsburgh, promoted a medicine called Banbar as being effective for diabetes. Banbar was an extract of horsetail weed.
How little things have changed. The homeopathic remedy equisetum is made from the horsetail plant, used to treat certain bladder conditions and “cure” bedwetting. If that wasn’t a big enough dose of nonsense for you, perhaps you’d be interested to know:
People who benefit most from using the homeopathic remedy equisetum are those who are bad-tempered as well as get exhausted quite easily.
Not even the shirt salesman went that far.
As Dr. Swann said of remedies like Banbar,
Products like these were, at minimum, useless remedies that picked the pocket of the user, but they could also be downright harmful.
Of course, he was describing products sold at the turn of the last century. Which, in a reasonable world, is exactly where we should have left homeopathy.
The FDA hearing
The two strongest witnesses at the hearing for reality-based medicine, as Mark Crislip would call it, were Michael DeDora of the Center for Inquiry, and Adriane Fugh-Berman, Associate Professor in the Department of Pharmacology and Physiology and the Department of Family Medicine at Georgetown University Medical Center.
Fugh-Berman is concerned about stocking homeopathic products on the shelves right next to other OTC drugs because, in her view, most consumers and medical professionals have no idea what homeopathic remedies are, don’t know they aren’t reviewed for safety and efficacy, and likely think they are dietary supplements or conventional OTC drugs. Nor does their dilution necessarily make them harmless, a point that was confirmed by Edward Krenzelok of the Rocky Mountain Poison and Drug Center in his remarks. For example, according to Fugh-Berman, Cold-EEZE contains 13.3 milligrams of zinc per lozenge. At recommended six lozenges a day, that’s about 80 milligrams of zinc a day, or ten times the RDA for adult females, eight times the RDA for males. As well, she said, products can contain snake venom, heavy metals, controlled substances, glandular extracts and other potentially dangerous ingredients.
Fugh-Bergman, who pointed out that the evidence for homeopathy is “between scant and nil,” wants to see the labels accurately describe the ingredients and their amounts in the same language used for other drugs and dietary supplements rather than the obscure vocabulary of homeopathy. She noted that, at some level of dilution, this would require expression of amounts in terms like “less than .01 nanograms.”
DeDora also spoke to the lack of evidence for homeopathy as well as its conflict with basic scientific principles, of which he said the FDA was “well aware.” He said, and I agree, that homeopathic drugs should have to meet the same standards as other drugs. That is what the law says, and that is what the FDA should do.
Both DeDora and Fugh-Berman suggested that, short of this, manufacturers put the equivalent of the Quack Miranda Warning on homeopathic drugs, a position with which I disagree for legal and other reasons. It doesn’t seem to dissuade people from taking dangerous supplements and I don’t find it consistent with Congress’s mandate that the FDA oversee the process by which manufacturers show safety and efficacy. Blowing off your duty to regulate simply by telling people you haven’t done your job seems less than satisfactory.
So much for the scientific point of view.
Appearing for the defense, if you will, was none other than Wayne Jonas, MD, of the Samueli Institute. He says we need more studies of homeopathy. Actually, we don’t, but I would have said the same thing if my salary was paid by a group that specializes in attracting government funds to study “alternative medicine.”
Amy Rothenberg, ND, whom you’ve met before here on SBM, spoke on behalf of the American Association of Naturopathic Physicians. The AANP believes the “FDA’s current regulatory approach to homeopathic products is working well.” I’ll bet they do. (Rothenberg is also trained in classical homeopathy.)
Like other naturopaths who spoke at the hearing, Rothenberg began her comments with an infomercial for naturopaths, pointing out their “extensive” classroom and clinical training, post-grad exams, and such. She talked about her use of homeopathy in her practice, including treating an autistic child. I find this interesting, because neither homeopaths or naturopaths are licensed in Massachusetts, where she practices, which means she is skating very close to, and perhaps over the line into, the practice of medicine.
Rothenberg was the only speaker who attempted to explain homeopathy’s purported mechanism of action which is, according to her, hormesis, that homeopathic canard David Gorski has already deconstructed.
The luminaries of the homeopathic industry were all there: representatives from the Homeopathic Pharmacopoeia Convention of the U.S., American Institute of Homeopathy, Homeopathic Academy of Naturopathic Physicians, Arizona Homeopathic and Integrative Medicine Association, National Center for Homeopathy, American Association of Homeopathic Pharmacists, Consumer Healthcare Products Association (a trade group), the Integrative Health Policy Association, North American Society of Homeopaths, and the Center for Education and Development of Clinical Homeopathy. The Kool-Aid flowed freely.
I can summarize all of their testimony fairly quickly:
1. Cherry-picked evidence
This included the famous Swiss Government Report, mentioned by Wayne Jonas, among others. Needless to say, other reports, such as the recent Australian National Health and Medical Research Council: Statement on Homeopathy or Evidence Check 2: Homeopathy from the UK were absent from pro-homeopathy discussion, save Jonas’s criticism of the former, which was, according to him, “very poorly done.”
An example from the comments of Tanya Kell, President of the North American Society of Homeopaths: Kell testified that:
there is a long, established record of both safety and efficacy in homeopathy. Homeopathic practice is based on hundreds of years of clinical experience by both physicians in this country and around the world.
Panelist and FDA regulatory attorney Elaine Lippman asked Kell what her data sources were “for that long established record particularly with respect to efficacy.”
Kell’s answer:
This is more a compilation of anecdotal evidence, so when I studies [sic] social work that was an accepted form of evidence, is just compiling anecdotal stories. [Actually, it’s not.]
Homeopathy has hundreds of millions of those, and you know, as our reputation grows, so does those anecdotal stories, and we feel that they are a legitimate source of data.
Translation: RCTs don’t apply to us.
And, from Bernardo Merizalde, MD, of the American Institute of Homeopathy:
We believe the true scientist does not discard available data because the theory doesn’t correspond to their prevailing world view.
Translation: The sciences of physics and chemistry are simply “world views” that can be ignored.
3. A whole lot of declarations that homeopathy is “safe” and “effective” and “it works”
Which just goes to show you: these people damn well know how to form a declarative sentence. They have it down. Totally. Good for their grammar teachers!
4. Explanations of the intricate process by which the HPCUS decides which homeopathic remedies are included in the HPUS
To which I say: GIGO.
Yet, Elaine Lippman was able to extract this confession from none other than Todd Hoover of the Homeopathic Pharmacopoeia Convention of the United States, the industry-controlled group that decides what homeopathic remedies are included in the Pharmacopoeia. She asked:
What is it about the FDA’s process of establishing efficacy through the gold standard, placebo-controlled, randomized clinical trials, what about that is inconsistent with homeopathic remedies?
To which Hoover replied:
There’s nothing inconsistent. Impractical might be a better word.
He later admitted that it is “certainly possible.” But then there was a big, fat qualifier to his admission. Why it is impractical?
The selection process and inclusion criteria that would be required might require a large population selection in order to find people who fit that particular remedy perfectly in order to demonstrate effectiveness for a given claim.
In other words, to do an RCT the homeopathic way, you would have to buy into the ridiculous nonsense of choosing remedies based on the patient’s quirky characteristics. Sound like more special pleading. In any event, this is not the way most people use homeopathy. About 95% of homeopathic products are OTC, so the user is selecting the remedy, not a homeopath.
Several other MDs testified in favor of homeopathy as well. Robert Dumont, MD, a pediatrician, said that homeopathic products were “exceptionally versatile and efficacious for many medical problems,” that he has “prescribed homeopathic medicines in the hospital for premature infants,” and used it for “nausea, vomiting and losing weight” in a patient undergoing chemotherapy. Another MD and classically trained homeopath, Karl Robinson, uses homeopathy for patients with hepatitis A. Younger Chug, MD, a pulmonologist, said he uses homeopathic products for asthma, chronic cough, croup and in situations where there is no effective drug, such as dyspnea due to vocal cord dysfunction. Another said homeopathy was “especially beneficial in our sickest patients.”
These physicians might just want to take a look at the Royal Australian College of General Practitioners recent “Position statement: homeopathy,” which includes this:
Medical practitioners should not practice homeopathy, refer patients to homeopathic practitioners, or recommend homeopathic products to their patients.
If this is the best evidence homeopathy has to offer, and the FDA decides to regulate homeopathic drugs in the same manner as all other OTC and prescription drugs (assuming it doesn’t allow homeopathic pseudoscience any place in the process), then homeopathy is indeed dead in the water, as FDA Law Blog suggests. The homeopathic industry is surely busy supplementing its poor showing before the FDA with written submissions. Speaking of which, we’ll now turn to the public comments.
Public comments
There are, as I mentioned, over 7,000 comments online. I have no idea how many more have been submitted in writing via snail mail. As I also mentioned, the FDA extended the deadline for comments until August 21, for reasons I cannot imagine. What’s more to be said?
I read through about 325 comments of the 7,000 plus (you’re welcome). They can be divided into three categories:
- A tiny percentage in favor of the FDA fulfilling its duty to regulate homeopathic drugs just like it regulates other drugs, some citing research in support of their position.
- Something along the lines of “you can have my homeopathic remedy when you peel it from my cold, dead fingers,” usually served up with a big dose of conspiracy theory, Big Pharma and government bashing, and Health Freedom, or HEALTH FREEDOM!!!
- Anecdotes demonstrating in the writer’s mind, with absolute certainty, that homeopathy “works.”
Boiron, the homeopathic remedy manufacturer, has been chumming up consumers to post their wonderful results online. What they’ve produced instead is a treasure trove of evidence that the public is being duped by the homeopathic industry. Based on my review, here is a list of diseases and conditions for which consumers are using homeopathy:
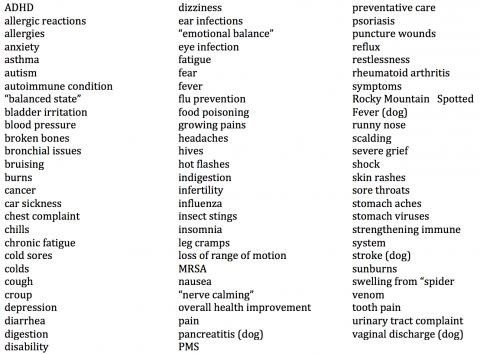
These consumers apparently have no understanding that the perceived “effectiveness” of homeopathic products could well be due to the natural course of disease, motivated reasoning, placebo responses, regression to the mean, confirmation bias, conditioning, the post hoc ergo propter hoc fallacy, or the effects of other treatments. In fact, from a scientific standpoint, these are the only plausible explanations for the putative effectiveness of homeopathic products.
These are exactly the sort of confounding factors that the premarket approval process is designed to weed out. By putting the onus on prescription and OTC drug manufacturers to demonstrate efficacy, this process ensures that consumers will not be purchasing remedies that actually do them no good and allows calculation of the true benefit of the product itself so that a proper risk-benefit analysis can be performed. As it now stands, the homeopathic industry is allowed to exploit other, plausible explanations for the perceived effectiveness of their products in order to sell an unnecessary product for its own financial gain.
One issue raised at the hearing was consumer confusion when homeopathic drugs are sold side-by-side with other OTC drugs. Homeopathy supporters pointed to the fact that these products are labeled “homeopathic,” but this begs the question. That means nothing if the consumer doesn’t understand what “homeopathic” means. Of course, to the homeopathic industry, it means that “like cures like” and that dilution combined with vigorous shaking makes the product more, rather than less, effective.
Consumers testifying at the hearing and commenting online parroted this explanation, ironically demonstrating they have no idea what “homeopathic” really means: that the product has been produced by a method that guarantees it will not work as advertised. One consumer repeated a version of the homeopathic industry’s attempts at reconciling their scientifically untenable “theory” with basic chemistry and physics: “we now know that water holds energetic information and the energy of each substance is stored within the homeopathic remedy.” Another said they are “effective through their energetic frequency and the effect they have on the cellular electrical energies.” Not only have consumers bought into the false claim that homeopathy “works,” they’ve swallowed the pseudoscience that “explains” its putative effectiveness.
What will the FDA do? I don’t know. If it wants to do the right thing, the FDA could impose the same requirements on homeopathic drugs as other drugs, which it has the authority to do. (And, if it had done this in the past, we wouldn’t find ourselves in the current situation.) But that carries some risk: homeopathic proponents might go running to their representatives in Congress to pass a law exempting homeopathic remedies from FDA oversight altogether. Or moving them to dietary supplement classification by redefining that term.
The most politically expedient thing to do may be requiring the homeopathic industry to label its OTC products like other OTC drugs, which would include a prohibition against making disease claims that are not supported by adequate evidence and stating ingredients in terms the average consumer has some chance of understanding. This would stop short of regulating them like other OTC drugs, but may take care of the problem without having to go there.
Blog archive: The FDA and homeopathic drug regulation (or lack thereof)
Science-Based Medicine
Homeopathic regulation diluted until no substance left
Should the FDA crack down on homeopathic “remedies”
How should the FDA regulate homeopathic remedies?
Respectful Insolence
Regulating the magic that is homeopathy: The sabotage of poor reporting and false balance

