One of the difficult things about science-based medicine is determining what is and isn’t quackery. While it is quite obvious that modalities such as homeopathy, acupuncture, reflexology, craniosacral therapy, Hulda Clark’s “zapper,” the Gerson therapy and Gonzalez protocol for cancer, and reiki (not to mention every other “energy healing” therapy) are the rankest quackery, there are lots of treatments that are harder to classify. Much of the time, these treatments that seemingly fall into a “gray area” are treatments that have shown promise in animals but have never been tested rigorously in humans or are based on scientific principles that sound reasonable but, again, have never been tested rigorously in humans. (Are you sensing a pattern here yet?) Often these therapies are promoted by true believers whose enthusiasm greatly outstrips the evidence base for their preferred treatment. Lately, I’ve been seeing just such a therapy being promoted around the usual social media sources, such as Facebook, Twitter, and the like. I’ve been meaning to write about it for a bit, but, as is so often the case with my Dug the Dog nature—squirrel!—other topics caught my attention.
I’m referring to a diet called the ketogenic diet, and an article that’s been making the rounds since last week entitled “Ketogenic diet beats chemo for almost all cancers, says Dr. Thomas Seyfried.” Of course, when I see a claim such as that, my first reaction is, “Show me the evidence.” My second reaction is, “Who is this guy?” Well, Dr. Seyfried is a professor of biology at Boston College, who’s pretty well published. He’s also working in a field that has gained new respectability over the last five to ten years, namely cancer metabolism, mainly thanks to a rediscovery of what Otto Warburg discovered over 80 years ago. What Warburg discovered was that many tumors rely on glycolysis for their energy even in environments with adequate oxygen for oxidative phosphorylation, which generates the bulk of the chemical energy used by cells. I described this phenomenon in more detail in a post I did four years ago about a drug that looks as though its anticancer properties come from its ability to reverse the Warburg effect.
What not to do if you want your hypothesis to be taken seriously
So on the surface, Dr. Seyfried’s argument that cancer is primarily a metabolic disease (an argument I’ll look at in more depth shortly) is well within the bounds of current oncologic science. Indeed, a few years ago it was all the rage, and I remember attending several sessions and lectures on the Warburg effect and cancer at the AACR meetings three or four years ago, although, oddly enough, I don’t recall as many the last couple of years. In any event, if that’s all I looked at, I probably would have shrugged my shoulders and moved on, as in, “Nothing to see here.” But there are quite a few red flags. The first red flag is a claim that a ketogenic diet can treat cancer better than chemotherapy. The second, even bigger, red flag is on Dr. Seyfried’s Boston College web page:
In addition, Dr. Seyfried has worked with noted alternative health advocate Dr. Mercola to provide a thought-provoking discussion on the benefits of a ketogenic diet. Dr. Mercola provides a thorough synopsis of the talk on his website, and also includes the original audio recording of their conversation.
Über-quack Dr. Mercola? Oh, dear. His evident pride at having been interviewed by Dr. Mercola does not reflect well on Dr. Seyfried’s critical thinking skills and knowledge of medicine. Dr. Mercola sells quackery. He has promoted antivaccine views, breast cancer pseudoscience, and the rankest cancer quackery, such as that of Tullio Simoncini, who believes that all cancer is a fungus and that baking soda is the way to treat it, and the Gerson therapy, which involves massive doses of supplements and, of course, twice-a-day coffee enemas. Seriously, this is not the sort of person a legitimate scientist wants to associate himself with—ever—if he wants to be taken seriously. I can see a naive researcher making a mistake and, not realizing who Dr. Mercola is, agreeing to an interview, but that’s the sort of thing that a reputable scientist would do his best to disavow and distance himself from.
Neither is the American College for Advancement in Medicine (ACAM), which bills itself as the “voice of integrative medicine,” where he’s given a major talk, the sort of organization a legitimate scientist wants to associate himself with if he wants to be taken seriously. Don’t believe me? Just peruse the ACAM website, where you will find lots of chelation therapy, including a program to “certify” in chelation therapy and detoxification, as well as other quackery. There’s a good reason that ACAM has appeared in many SBM posts throughout the years, and not in a favorable light. I emphasize again, this is not an organization with which a scientist who wishes to be taken seriously by oncologists associates himself.
Also, if a scientist wishes to be taken seriously, he shouldn’t say things like this:
The low-carb, high-fat ketogenic diet can replace chemotherapy and radiation for even the deadliest of cancers, said Dr. Thomas Seyfried, a leading cancer researcher and professor at Boston College.
In an exclusive interview, Dr. Seyfried discussed why the ketogenic diet has not been embraced by the medical community to treat cancer despite its proven track record both clinically and anecdotally.
“The reason why the ketogenic diet is not being prescribed to treat cancer is purely economical,” said Dr. Seyfried, author of Cancer as a Metabolic Disease. “Cancer is big business. There are more people making a living off cancer than there are dying of it.”
And don’t associate yourself with Ralph Moss, the number one promoter of laetrile quackery and make easily refuted claims such as the claim that “chemo and radiation do not cure cancer or extend life, although cancer physicians often make this claim” and that radiation “often does more harm than good to the patient.” Given that all Dr. Seyfried has is a couple of case studies as clinical support for his treatment (see below) and I can produce reams of studies over nearly 50 years demonstrating that chemotherapy can cure specific cancers and prolong life when used appropriately, the “2% gambit” notwithstanding, it’s not a winning proposition, and it sure doesn’t help your credibility to use the language of cancer quacks to promote your idea.
So, what, exactly is Dr. Seyfried’s hypothesis?
Cancer as a metabolic disease
Red flags or no red flags, it is, of course, possible that Dr. Seyfried is on to something and has let his enthusiasm overwhelm his judgment with respect to whom he associates with and the sorts of statements he makes, many of which sound as though they could have come from Stanislaw Burzynski, Ralph Moss, or Joe Mercola. In actuality, he isn’t totally wrong, but he isn’t totally right, either. As is typical of someone without a medical background, in particular an oncology background, he is, basically, putting the cart before the horse, as you will see.
In his talk, Dr. Seyfried begins with what he refers to as a “provocative question”: Is cancer a genetic or metabolic disease? Actually, whether he realizes it or not, his question is not quite as provocative as he thinks it is, nor is the answer anywhere near as clear-cut as he thinks it is or as he characterizes oncologists and cancer researchers as thinking it is. I’ll tell you what I think the answer to the question is after I’ve discussed Dr. Seyfried’s hypothesis. In the meantime, not surprisingly, his answer is that cancer is a metabolic disease, while everyone else’s answer—according to him, at least—is that it is a genetic disease, making him the brave maverick doctor, who says things like:
The current view now, without any question, is that cancer is a genetic disease. If you go on the National Cancer Institute website or you read any of the major articles published in Nature and Science, often the articles will start with, “Cancer is a genetic disease.” I think that this has become dogma.
Except that it really isn’t, at least not anymore. If you do a Pubmed search on “targeting cancer metabolism,” which is what Dr. Seyfried is talking about, you’ll find over 22,000 articles, with over 3,000 in 2013 alone, with a sharply increasing curve since 2000 that only now appears to be leveling off. A search on “cancer metabolism” brings up 369,000 references, with 28,000 in 2013 alone. Cancer metabolism is an incredibly important topic in cancer research and has been for several years now, and finding means of targeting the common metabolic abnormalities exhibited by cancer cells is currently a hot area of research. From my perspective, Dr. Seyfried is exaggerating how hostile the cancer research community is towards metabolism as an important, possibly critical, driver of cancer, although, to be fair, one prominent cancer researcher, Robert Weinberg, has been very skeptical. To me, Seyfried just appears unhappy that genetics is currently thought—for good reasons, I might add—to be the primary driver of most cancers. Note that I intentionally used such phrasing, because Dr. Seyfried, in my readings, appears all too often to speak of “cancer” as if it were a monolithic single disease. As I’ve pointed out many times before, it’s not. Indeed, only approximately 60-90% of cancers demonstrate the Warburg effect.
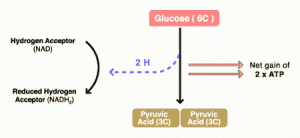
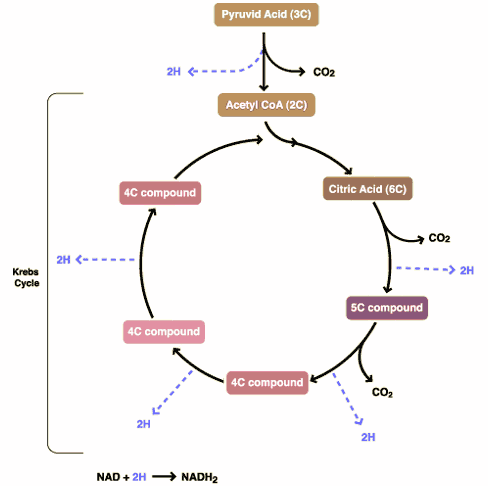
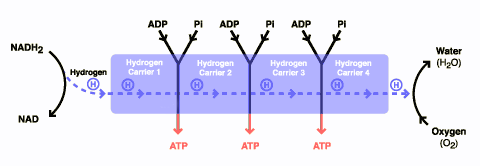
There are three components to glucose metabolism: glycolysis, which feeds the Krebs citric acid cycle, which in turn feeds oxidative phosphorylation. I show them below in simplified illustrations:
The issue with the Warburg effect is that it leads to a shift in metabolism that favors glycolysis. As a result of this shift, tumor cells tend to use a lot more glucose than normal cells because glycolysis is much less efficient at converting glucose into ATP molecules used for cellular energy than oxidative phosphorylation. One reason that this is thought to provide a growth advantage to cancer cells is because oxidative phosphorylation requires oxygen while glycolysis does not and cancers frequently outgrow their blood supply such that they often live and grow in tissue spaces where there is not much oxygen. In any case, the avidity of cancer cells for glucose has been known a long time and is the basis for positron emission tomography (PET) scanning, where a radiolabeled derivative of glucose is the most commonly used tracer for exactly that reason: Tumor cells take it up much more avidly than do normal cells, leading to ugly black blobs (old-fashioned PET scans alone) or pretty bright blobs (PET-CT) where there are tumor masses in the scans.
The idea behind ketogenic diets is very simple. If glucose is the primary fuel for cancer, then lower carbohydrate intake and replace carbohydrates with other sources of fuel, such as fats, in order to push the body’s metabolism into ketosis. It actually turns out that ketogenic diets are probably useful in the treatment of intractable epileptic seizures in children. Unfortunately, their mechanism of action in preventing seizures is unclear, although four potential mechanisms, including carbohydrate reduction, activation of ATP-sensitive potassium channels by mitochondrial metabolism, inhibition of the mammalian target of rapamycin (mTOR) pathway, or inhibition of glutamatergic synaptic transmission (glutamate as a neurotransmitter), have been proposed. Interestingly, the mTOR pathway is an important signaling pathway in many cancers that couples energy and nutrient abundance to the execution of cell growth and division, owing to the ability of TOR protein kinase to simultaneously sense energy, nutrients and stress and growth factors. It’s a commonly overactive signaling pathway in cancer.
It’s also interesting that the cancers used to produce the basic science cited by Dr. Seyfried are virtually all brain cancers and that virtually all the evidence comes from rodent tumor models. For one thing, if there is a tumor type that exhibits the Warburg effect and a high degree of metabolic derangement, it’s brain tumors. It’s no coincidence that dichloroacetate was first tested in brain tumors. In this study, VM/Dk mice were used, and a mouse histiocytoma cell line resembling human glioblastoma multiforme with macrophage/microglial properties derived from that same mouse strain (VM-M3) was implanted subcutaneously. This cell line has the property of metastasizing quickly and widely when implanted under the skin and allowed to grow, which actually makes it not very much like brain tumors, which seldom metastasize and usually kill through local invasion and taking up increasing volume in the closed space of the skull, something the brain most definitely does not like. The results showed that a ketogenic diet increased mean survival time by over 56%, while a combination of a ketogenic diet and hyperbaric oxygen therapy (HBOT) increased survival time 78%. The result is interesting, but it is a mouse tumor model, not a human tumor model, and that makes its applicability to humans tenuous, particularly given the nature of the murine tumor, but probably worth investigating further.
Another mouse study cited by Seyfried is one in which dietary restriction was reported to promote vessel maturation in a mouse astrocytoma model. Given that tumor angiogenesis is one of my scientific interests and I have a fair number of publications on the topic, I was interested. Unfortunately, I ended up being disappointed. This was another syngeneic model (i.e., a mouse tumor implanted in mice of the same strain from which the tumor was isolated as a cell line, like the one discussed above). Although it showed increased tumor vessel maturation (which is one mechanism by which inhibitors of angiogenesis work), I wasn’t quite convinced, because there was a distinct lack of quantification of the phenomenon, and the microscopy appears not to have been blinded, something that’s critical to avoid unconscious bias in the results. It’s not surprising that this result, which, if more convincing evidence had been obtained, could easily have appeared in Cancer Research, was published in a low tier journal. It’s an OK study, but not fantastic. Certainly it didn’t lead me to smacking myself in forehead and saying, “Of course!”
Throughout his talks, both here and elsewhere, Dr. Seyfried presents mouse studies that are interesting and suggestive that there might be something to this whole ketogenic diet thing, at least in brain tumors, such as this one. However, this is what we in the oncology biz would call pretty preliminary data, worthy of further investigation but not supporting the grandiose claims that Dr. Seyfried makes.
We need more beef. We need clinical studies. Unfortunately, they’re in short supply.
Clinical evidence for ketogenic diets as a cancer treatment
It’s not as though Dr. Seyfried doesn’t cite clinical evidence. It’s just that the evidence is so darned thin and unconvincing thus far. For instance, in this talk, the first study he presents is a very small case series (two patients, actually) performed in 1995 in which two girls with inoperable astrocytomas were placed on a ketogenic diet in order to “determine if a ketogenic state would decrease glucose availability to certain tumors, thereby potentially impairing tumor metabolism without adversely affecting the patient’s overall nutritional status.” Interestingly (to me, at least) these case reports came from University Hospitals of Cleveland, where I did my general surgery residency. In fact, I was still there in 1995. Unfortunately, I don’t have access to the journal back to 1995; so I’m stuck with just the abstract. However, the abstract is pretty clear:
Within 7 days of initiating the ketogenic diet, blood glucose levels declined to low-normal levels and blood ketones were elevated twenty to thirty fold. Results of PET scans indicated a 21.8% average decrease in glucose uptake at the tumor site in both subjects. One patient exhibited significant clinical improvements in mood and new skill development during the study. She continued the ketogenic diet for an additional twelve months, remaining free of disease progression.
One notes that the patient who didn’t survive 12 months wasn’t much mentioned; so I assume she didn’t demonstrate any clinical improvement. In any case, this study doesn’t really show anything, other than that a ketogenic diet might decrease glucose uptake in some brain tumors. It’s like a Burzynski case report, in which we have no idea whether the patient did better than expected because of the intervention or because she had less aggressive disease.
The next case report is from 2010. It describes the case of a 65-year-old woman who presented with progressive memory loss, chronic headaches, nausea, and a right hemisphere multi-centric tumor seen with magnetic resonance imaging (MRI). Following incomplete surgical resection, the patient was diagnosed with glioblastoma multiforme (GBM). Now here’s the kicker: The patient underwent standard therapy plus the ketogenic diet. A day after her surgery, she underwent a two-day fast, followed by a three day fast beginning a week after surgery, followed by a restricted ketogenic diet (only 600 Cal/day). Three weeks after her surgery (and two weeks after starting the ketogenic diet) she began standard of care treatment, concomitant radiation plus chemotherapy (temozolomide), “according to standard procedures,” which lasted six weeks. The patient also had a gene mutation in her tumor that produces increased sensitivity to temozolomide. The conclusion? Fortunately for the patient, she had what appears to have been a complete response, after which she went on a less restrictive ketogenic diet. Unfortunately, the patient recurred eight months later. By that point, the patient was off of the ketogenic diet. The authors’ conclusion? Because it was “unlikely” that the tumor would have responded this well on standard therapy alone, it must have been adding the ketogenic diet that done it. Worse, in the talk, Dr. Seyfried strongly implies that the tumor recurred because she had gone off the ketogenic diet two and a half months before her recurrence.
Irritatingly, during the same talk, Dr. Seyfried refers to having done a “biopsy” on the GBM when the case report clearly says that the patient underwent a partial excision of the temporal pole with incomplete debulking of the tumor, which is a different thing. When a surgeon tries to debulk a tumor, he is trying to remove as much of it as possible. When a surgeon biopsies a tumor, he is trying only to get enough tissue to make a diagnosis. He also heaps scorn on the hospital for insisting that the patient undergo standard of care therapy, clearly demonstrating that he has no understanding of clinical trial ethics. What most likely happened with this patient is that the debulking was significant, and the remaining tumor was small enough to be eliminated by the combined chemotherapy and radiation therapy—at least to the point of no longer being detectable on PET scan. Also, just because the diet appears to have decreased glucose uptake by the tumor doesn’t mean that the tumor was dying. In fact, it might have even made the PET scan less sensitive to whatever remaining viable tumor cells might still have been around, a possibility that I don’t see Dr. Seyfried as having considered.
There are other studies, but little or nothing in the way of randomized clinical trials. For instance, a recent retrospective study of 53 patients, of whom only six followed a ketogenic diet while being treated for GBM, concluded that the diet was safe, but no suggestion of efficacy was noted. More recently, a German group examined the effect of a ketogenic diet on 16 patients with advanced cancer of various types who had exhausted all therapeutic options. The treatment didn’t result in any serious side effects, although subjects found it very difficult to maintain the diet, particularly in the context of family life. Only five were able to complete the three month treatment period, and it was reported that these five didn’t have progression while on the diet. Of the remaining 11, two died early, one was unable to tolerate the diet and dropped out very quickly, two dropped out for personal reasons, one couldn’t continue the diet for more than a month and three had disease progression within less than 2 months of starting the diet and one dropped out to resume chemotherapy. As a whole, this study was well-nigh uninterpretable due to the different kinds of cancer, other than to conclude that less than 50% of patients with advanced cancer could adhere to the diet, and that those who could generally had no significant side effects. Of course, it’s unclear whether the diet helped the five who could adhere to it or whether those who adhered to it could do so because they had more indolent, less aggressive disease.
None of this stops Dr. Seyfried from concluding:
- Preclinical and case report studies indicated that the restricted ketogenic diet (R-KD) can be an effective “metabolic therapy” for managing malignant brain cancer in children and adults.
- The therapeutic effects of the R-KD against brain cancer can be enhanced when combined with drugs or HBOT that also target energy metabolism.
Uh, no. Not exactly. Preclinical experiments are intriguing but fairly limited in applicability, and the case reports demonstrate nothing of the sort. There’s more to Dr. Seyfried’s hypothesis, for example, his idea that metastatic cancer comes about because of alterations in glutamine metabolism, but unfortunately he appears to misunderstand the genetics of metastasis when he bases part of his conclusion on observations that metastatic cancers often have the same genetic derangements as the primary tumor. It’s been a longstanding question whether clones of tumor cells possess the ability to metastasize as an intrinsic part of the process of becoming cancer cells or whether they acquire it later. Given that evolution is a major force driving cancer cells to become more invasive and that tumors are very heterogeneous, full of lots of different clones with different sets of genetic mutations, Dr. Seyfried’s hypothesis is at best simplistic. Also disappointingly, the evidence for any diet as a treatment for cancer is weak at best.
Putting the cart before the horse
Clearly, ketogenic diets are not ready for prime time as a treatment for cancer, either alone or in combination with conventional therapy. Unfortunately, that hasn’t stopped it from being touted by all manner of alternative cancer practitioners (i.e., quacks) and others as a cancer cure that “they” don’t want you to know about or saying things like, “…it’s nothing short of medical malpractice and negligence to fail to integrate this type of dietary strategy into a patient’s cancer treatment plan,” as Joe Mercola did. Dr. Seyfried himself has contributed to the hyperbole quite a bit as well. For example:
These studies are all in combination with either radiation or chemotherapy. My preference is to start metabolic therapy with GBM (glioblastoma multiforme). This is a devastating type of brain cancer. Metabolic therapy with a restricted KD could be done with a few tumors where you know the conventional standard of care doesn’t work at all. You would choose those kinds of patients and do a clinical trial based on historical controls and see what the outcome would be and see if you could get some level of survival that would match or be better than the conventional standard of care.
Regular readers of SBM should know the problem with this sort of approach. No IRB worth its salt would approve such a trial because it would be ethically dubious, but, even worse, it would be ethically dubious and it wouldn’t really tell us anything unless those few patients either had near-miraculous responses or died very quickly. Anything else would simply tell us that the diet is probably doing no harm. More numbers would be needed, particularly if the comparison is to historical controls, to get even an inkling of whether there might be benefit. In that case, you might as well do a proper phase I/II clinical trial, which is what is happening. For instance:
- Calorie-restricted, Ketogenic Diet and Transient Fasting During Reirradiation for Patients With Recurrent Glioblastoma (ERGO2), a randomized clinical trial designed to evaluate whether a calorie-restricted, ketogenic diet and transient fasting can enhance the efficacy of reirradiation in patients with recurrent glioblastoma.
- Pilot Study of a Metabolic Nutritional Therapy for the Management of Primary Brain Tumors (Ketones), a phase I pilot study in my neck of the woods (at least in my state) looking at the same sort of diet.
- Ketogenic Diet as Adjunctive Treatment in Refractory/End-stage Glioblastoma Multiforme: a Pilot Study, a small pilot study designed to examine the effect of a ketogenic diet in refractory GBM being treated with Avastin.
- Ketogenic Diet With Radiation and Chemotherapy for Newly Diagnosed Glioblastoma, a phase I/II trial designed to test whether a ketogenic diet enhances the efficacy of radiation and chemotherapy.
In other words, clinical data should be rolling in fairly soon, and that’s a good thing. In the meantime Dr. Seyfried and other advocates who so passionately believe that ketogenic diets will greatly help patients with brain cancer do no one any favors by claiming unequivocally that cancer is a metabolic disease and saying that ketogenic diets are more beneficial than chemotherapy for patients with brain tumors.
This brings me back to the question of whether cancer is a metabolic disease or a genetic disease, the answer to which I promised early on. The likely answer? It’s both! Indeed, a “chicken or the egg” argument continues about whether it is the metabolic abnormalities that cause the mutations observed in cancer cells or whether it is the mutations that produce the metabolic abnormalities. Most likely, it’s a little of both, the exact proportion of which depending upon the tumor cell, that combine in an unholy synergistic circle to drive cancer cells to be more and more abnormal and aggressive. Moreover, cancer is about far more than just the genomics or the metabolism of cancer cells. It’s also the immune system and the tumor microenvironment (the cells and connective tissue in which tumors arise and grow). As I’ve said time and time and time again, cancer is complicated, real complicated. The relative contributions of genetic mutations, metabolic derangements, immune cell dysfunction, and influences of the microenvironment are likely to vary depending upon the type of tumor and, as a consequence, require different treatments. In the end, as with many hyped cancer cures, the ketogenic diet might be helpful for some tumors and almost certainly won’t be helpful for others. Dr. Seyfried might be on to something, but he’s gone a bit off the deep end in apparently thinking that he’s found out something about cancer that no one else takes seriously—or has even thought of before.