Tag: clinical trials
Pegcetacoplan (Syfovre™) for macular degeneration: an update
SBM's guest contributor and ophthalmologist, Dr. David Weinberg, provides an update on the phase 3 trials of pegcetacoplan for macular degeneration. The results are still disappointing.
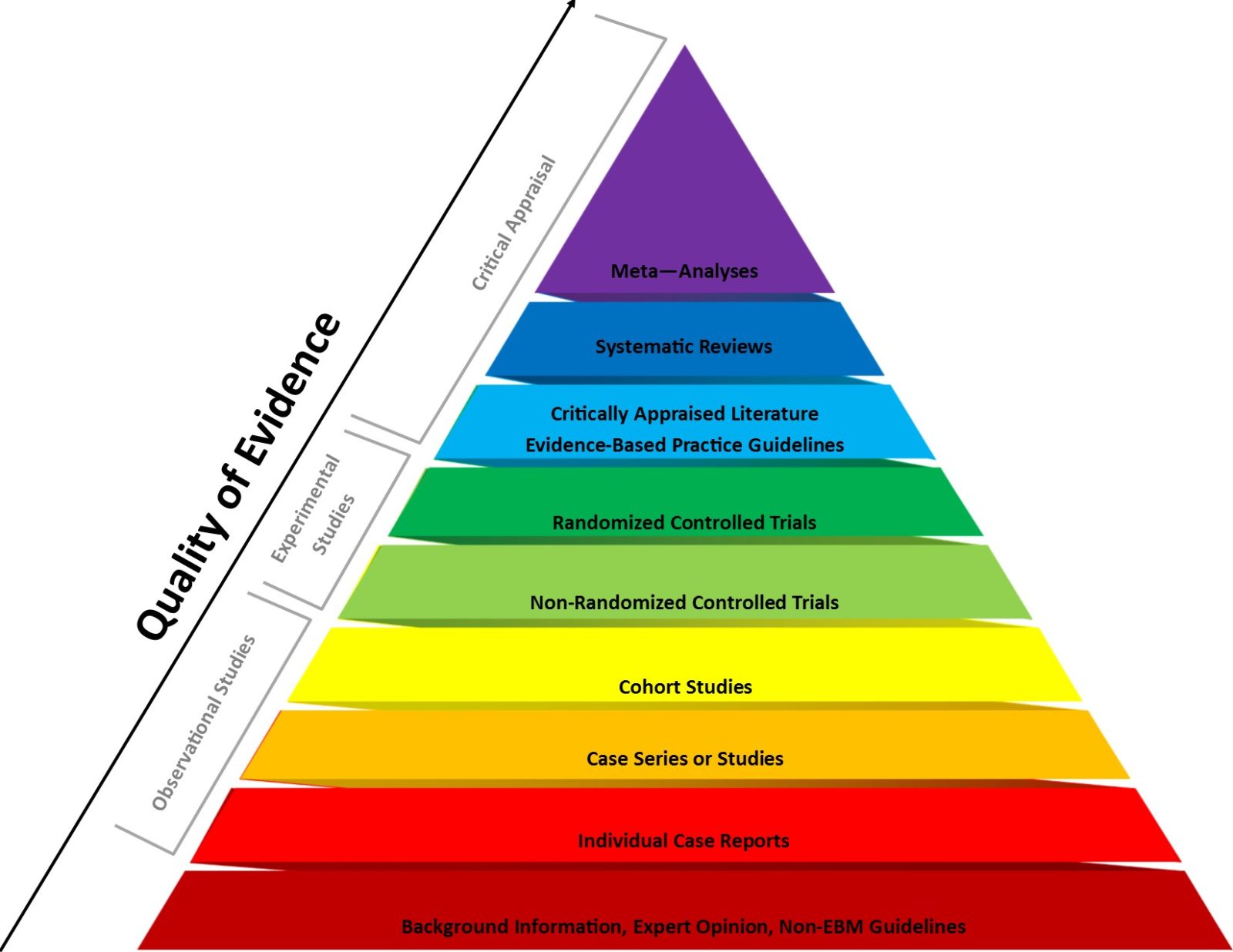
2023: The year that the evidence-based medicine (EBM) paradigm was weaponized against vaccines and public health
Evidence-based medicine (EBM) has been a very useful paradigm for assessing evidence in medicine. However, like any other framework, it can be misused, particularly when fundamentalist EBM methodolatry leads to its inappropriate application to questions for which it is ill-suited, a misuse that has been weaponized against public health during the pandemic.

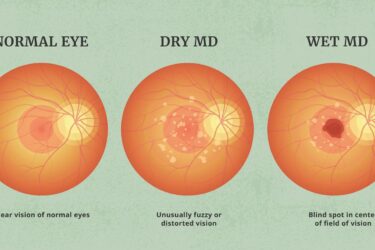
Pegcetacoplan, a new treatment for macular degeneration
FDA approves a new treatment for macular degeneration: the good, the bad, and the disappointing.
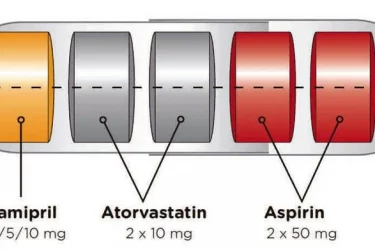
The Polypill Comes of Age
The polypill is effective for secondary prevention of cardiovascular disease. By combining drugs in a single pill, it improves convenience and compliance.
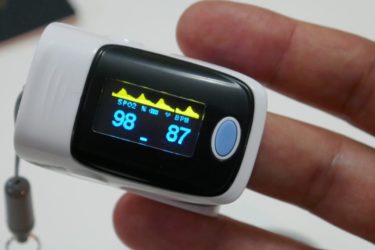
COVID-19 puts the spotlight on an unexpected racial disparity in health care
Evidence increasingly suggests that pulse oximeters, the little finger clips that measure blood oxygen, overestimate the blood oxygenation in Black patients. It's a problem that's been discussed a long time that took a pandemic to bring to public consciousness. How can SBM decrease or eliminate such healthcare disparities?

Gender-Affirming Care is Not Experimental, Part II
A lot of the "facts" about providing healthcare to transgender youth turn out to be not actually facts. We present here a summary of the evidence relating to transition-related health care for transgender adolescents.
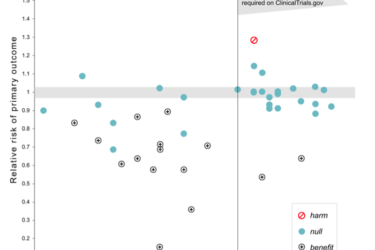
Homeopathy and Pre-Registered Trials
Preregistering clinical trials is a great idea, but we have to actually track registration.

Signos Sells a Continuous Glucose Monitor, But Not to Diabetics
Signos is asking customers to pay for the privilege of testing their glucose monitoring system.
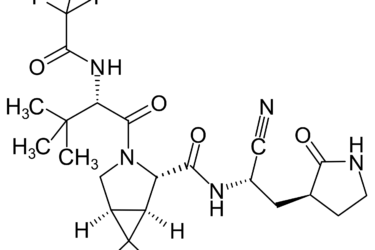
Pfizer’s new COVID-19 protease inhibitor drug is not just “repackaged ivermectin”
Pfizer recently announced that its new protease inhibitor-based drug was 89% effective in preventing hospitalization due to COVID-19 and it is seeking an emergency use authorization for it from the FDA. Antivaxxers claim that ivermectin targets the same protease and is being "suppressed" to protect Pfizer's profits from the new drug. What's the real story? Hint: Antivaxxers took a grain of truth...