Tag: Dietary Supplement Health and Education Act of 1994

FDA warns companies selling illegal hangover remedies
The FDA recently warned seven companies not to claim that their dietary supplements can prevent, treat, or cure a hangover, because only FDA-approved drugs can make such claims. The agency also warned that NAC, a popular supplement ingredient, cannot legally be used in dietary supplements.

FDA Decision on Oleandra
FDA rejects the application for oleandrin as a new dietary ingredient, but flaws in the regulations remain.

FDA: No CBD in dietary supplements or foods for now, but let’s talk
The FDA reminds everyone that (no matter what your state says) CBD is not a legal ingredient in dietary supplements and foods. The agency is willing to explore changes to the law but unproven claims for CBD health benefits, such cancer cures, will not be tolerated.

FDA promises industry-friendly “modernization” of dietary supplement regulation
The FDA promises the "most significant modernization of dietary supplement regulation" in 25 years while maintaining its industry-friendly regulatory scheme.

Drugs in your supplements
Supplements are a billion-dollar business, but quality control is questionable. A new study shows that supplements may be adulterated with unlabelled prescription drugs.

The Congressional Dietary Supplement Caucus
The Congressional Dietary Supplement Caucus, an officially-recognized Congressional Membership Organization, operates as an in-house mouthpiece for the dietary supplement industry. Both the caucus and the rules allowing it should be reformed to prohibit this.

The Supplement Con
A new article in Business Insider challenges the major narrative promoted by the supplement industry - that supplements are safe, effective, natural, and actually in the bottle. If we are lucky, this may mark a the start of a sea change in how Americans see supplements.

Increase In Supplement Poisonings
Current supplement regulations in the US (and many countries) are overtly anti-consumer and pro-industry, and are the direct result of aggressive industry lobbying and having powerful senators in their pocket. The rise in calls to poison control for supplements are just one manifestation of this situation.
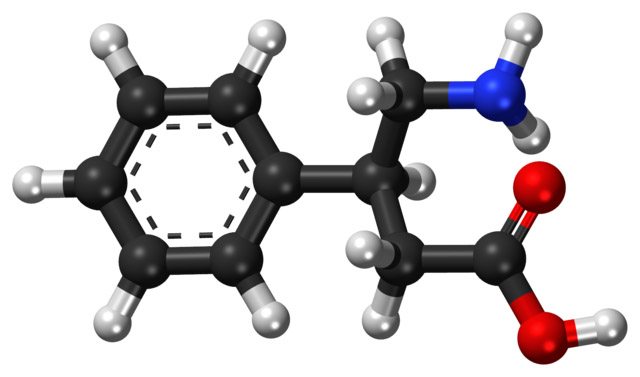
Phenibut Is Neither Proven Nor Safe As A Prosocial Wonder Drug
Editor’s note: With Mark Crislip away on yet another vacation, we present an inaugural guest post from Abby Campbell, a practicing MD, Ph.D and contributor at HealthyButSmart.com. Welcome Abby! On average for the past year, phenibut has been typed into google 49,500 times a month. Phenibut is a supposed wonder drug that claims to promote sociability and lessen anxiety. When people run...
Natural Health Products: Loosely regulated, little evidence of benefit, and an industry intent on preserving the status quo
This week’s post will revisit a topic I recently covered, but it’s time-sensitive and needs your input. Health Canada, the Canadian equivalent to the US Food and Drugs Administration, is considering revisions to the way in which it regulates dietary supplements, which are called “natural health products” in Canada. It is rare that a regulator acknowledges that a regulatory system isn’t working,...

