Tag: FDA

The Truth vs. Alex Jones: How the DSHEA of 1994 gave conspiracy mongers the means to fund their empires
As the HBO documentary The Truth vs. Alex Jones shows, Alex Jones promoted the conspiracy theory that the Sandy Hook massacre was a hoax to sell his supplement line. It's a model that many Internet conspiracy theorists use, like Mike Adams. Did the DSHEA help create Alex Jones and the modern conspiracy industry?
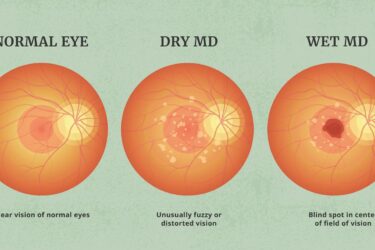
Pegcetacoplan (Syfovre™) for macular degeneration: an update
SBM's guest contributor and ophthalmologist, Dr. David Weinberg, provides an update on the phase 3 trials of pegcetacoplan for macular degeneration. The results are still disappointing.
RFK Jr. and his “I’m not anti-vaccine” rejoinder to being confronted with his past antivax statements: A primer
On Friday, CNN host Kasie Hunt interviewed antivax presidential candidate Robert F. Kennedy, Jr. Although she did better than most journalists confronting him for his past antivax statements in that she played a clip of one of his antivax statements, she clearly hadn't anticipated his response, which should have been very predictable given that he's been using it for at least 15...

What the heck happened to The BMJ? (2023 version)
The BMJ, once a bastion of evidence-based medicine, has become disturbingly susceptible to publishing biased "investigations" that feed antivax narratives. Its latest report on VAERS by Jennifer Block, who in the past has defended Gwyneth Paltrow and Goop and whose history is not one of supporting science, is just another example of this deterioration.

Decongestant doesn’t work, concludes FDA advisory committee
An FDA advisory committee has concluded that phenylephrine, a popular decongestant in cough and cold remedies, is ineffective.

The (Cold) Drugs Don’t Work
The FDA continues to permit the inclusion of an ineffective ingredient in hundreds of cough and cold products.

After 15 years of SBM: Lessons learned and what the future holds
Last week, Dr. Novella discussed what SBM has accomplished over the last 15 years. I'm going to discuss lessons learned, what has changed, and remaining huge challenges. Unfortunately, after the pandemic, our position in 2022 reminds me even more than ever of Aragorn at the Black Gate of Mordor, but that does not mean things are hopeless.

