Tag: Food and Drug Administration

Adios Aduhelm
The controversial and never-proven-effective drug to treat Alzheimer's disease, aducanumab (Aduhelm) has been discontinued.
The Pfizer COVID-19 vaccine doesn’t prevent transmission: Antivax disinformation goes viral again
Last week, antivaxxers were all over social media after Tucker Carlson touted a "revelation" that the phase 3 clinical trial used to support licensure of the Pfizer COVID-19 vaccine didn't examine its ability to block transmission as meaning that its inability to block transmission had been "covered up". It wasn't, and antivaxxers are ignoring everything we've learned over the last two years...
FDA Approves Controversial ALS Drug – Relyvrio
A close look a the FDA approval of Relyvrio.

Medicare and Medicaid place restrictions on new Alzheimer’s drug, Aduhelm (aducanumab)
An important decision has been made about a controversial new drug to treat Alzheimer's disease.

Virtual Reality Therapy
Are virtual reality headsets a valid tool for treating back pain? Maybe.

What the heck happened to The BMJ?
Last week, The BMJ published an "exposé" by Paul Thacker alleging patient unblinding, data falsification, and other wrongdoing by a company running three sites for the massive clinical trial of the Pfizer COVID-19 vaccine. It was a highly biased story embraced by antivaxxers, with a deceptively framed narrative and claims not placed into proper context, leading me to look into the broader...
Yes, the FDA really HAS given full approval to the Pfizer/BioNTech COVID-19 vaccine
After the FDA announcement a week ago that Comirnaty, the COVID-19 mRNA vaccine developed by BioNTech and Pfizer, had been approved, it took less than a day for antivaxxers to spin a conspiracy theory claiming that the FDA hadn't really approved the Pfizer vaccine at all and that Comirnaty wasn't available, all to protect Pfizer from liability. It's a superficially plausible conspiracy...

Court overturns FDA ban on electric shock device used on special needs students
An FDA ban on using electric shock devices to control behavior in special needs students, a method deemed "torture" in a U.N. report, was overturned in a flawed court decision. The FDA should appeal.
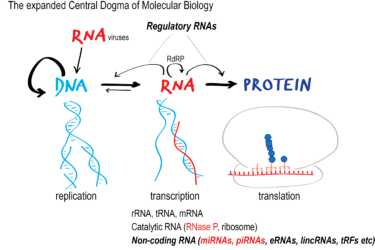
The latest antivax false claim: mRNA vaccines against COVID-19 are not vaccines but “medical devices” or “gene therapy”
There's a new antivaccine talking point in town, and it's just as much disinformation as other antivaccine talking points. It's the claim that mRNA COVID-19 vaccines are not really vaccines but "medical devices," "gene therapy," or "experimental biologics" and that they were falsely classified as vaccines in order to bypass safety testing. Here, we discuss why this claim is utter nonsense based...

FDA and FTC issue more warning letters citing products and services making illegal COVID claims
The FDA and FTC have issued hundreds of warnings to companies selling products and services claiming, without adequate evidence, that they can prevent or treat COVID-19, but the possibility of government action doesn't seem to be a deterrence.

