Tag: Pharmaceuticals
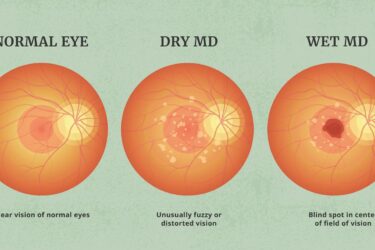
Pegcetacoplan (Syfovre™) for macular degeneration: an update
SBM's guest contributor and ophthalmologist, Dr. David Weinberg, provides an update on the phase 3 trials of pegcetacoplan for macular degeneration. The results are still disappointing.

Importing drugs from Canada won’t lower drug prices for Americans
The Trump administration has approved the bulk importation of prescription drugs from Canada. But Canada isn't on board with this plan, and it's not going to reduce drug prices for Americans.

Are those “inactive” ingredients in my medicine really inactive?
Drugs and supplements contain dozens of inactive ingredients. Is this a concern to those with allergies and sensitivities?

What’s all that other stuff in my medicine?
Are all those extra ingredients in your medicine or supplement, like fillers and coatings, something to be concerned about?
Donald Trump versus the FDA: Is the standard of evidence for drug approval actually too low rather than too high?
All of the candidates being considered by President Trump for FDA Commissioner believe that the FDA is too strict in its standards for approving new drugs. In a commentary in Nature last week, two bioethicists argued that, at least in terms of preclinical data, the standard of evidence is actually too low. Which is correct?

Drug therapy is still sending too many people to the emergency department
Prescription drugs continue to send thousands to the emergency room every year. Many of these adverse drug events are predictable and avoidable.
Whither the randomized controlled clinical trial?
With the rise of precision medicine and genomics, the conventional randomized clinical trial appears more and more outdated. Fortunately, clinical trials are evolving, but will it be enough to incorporate the numerous advances in "-omic" medicine in a rigorous scientific manner to benefit patients?
Legislators want “pharmaceutical cost transparency”. Are they asking the wrong question?
If science-based medicine is unaffordable, then your care won’t be science-based. Prescription drug costs are one of the biggest concerns in health care today. There seems to be no upper limit on prices, with some new treatments costing over $1,000 per day. The arrival of new drugs to treat (and cure) hepatitis C has created a perfect pharmaceutical storm: highly effective treatments,...
Cancer prevention: The forgotten stepchild of cancer research?
The New York Times has been periodically running a series about the “40 years’ war” on cancer, with most articles by Gina Kolata. I’ve touched on this series before, liking some parts of it, while others not so much. In particular, I criticized an article one article that I thought to be so misguided about how the NIH grant system leads researchers...


