Do you take a vitamin or dietary supplement? It’s increasingly likely that you do, as over half of all American adults took some sort of supplement over the past 30 days. Now there’s evidence to suggest that about one-third of all Americans are taking supplements and prescription drug at the same time, which is renewing questions about risks and benefits. The same study reveals that combining supplements and prescription drugs is more common among those with certain medical conditions, compared to those without.
Many of us supplement in the absence of evidence of benefit, or even medical need. For example, there is little persuasive evidence to suggest that routine supplementation with products like multivitamins is necessary. There are exceptions of course: Those potentially becoming pregnant, those on dietary restrictions (e.g., vegans), and those with demonstrable medical need are among the cases where there is a clear benefit to vitamin supplementation, for example. The majority of us take supplements, like multivitamins, for “insurance” rather than because we have a deficiency or medical need. The evidence for non-vitamin supplements, like herbal products, is just as questionable as it is for vitamins, with few products showing meaningful health benefits. Ultimately decisions about supplements come down to evaluations of risk and benefits. Since I started working as a pharmacist, I’ve always cautioned consumers about the quality concerns and efficacy with herbal products and supplements, and the resultant risks that make me very hesitant to suggest their routine use – especially when they’re combined with prescription drugs. Yet the evidence suggests that it’s occurring – with increasing frequency.
The supplement trend
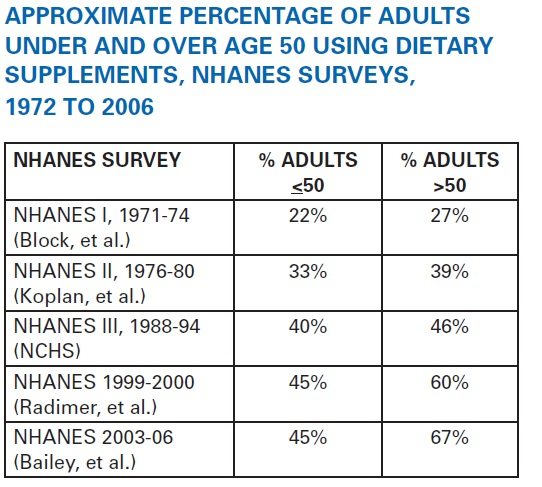
I’ve covered this in a past post, but it’s worth reflecting on the growing habit of consuming dietary supplements – 40 years of data from the National Health and Nutrition Examination Survey (NHANES) shows a doubling of use since the 1970’s:
Source: Council for Responsible Nutrition
With these numbers, it should be no surprise that supplements are now big business: this is a $30-billion-per-year (USD, 2011) industry with little regulatory oversight. The current marketplace is now rife with exaggerated claims and few products backed by credible evidence. And importantly, dietary supplements are not held and inspected to the same manufacturing and quality standards as pharmaceutical drugs – so there are persistent and worrying questions about the quality of products sold as supplements.
The quality question
Surveys suggest the vast majority of Americans are confident in the safety, quality, and effectiveness of these products. Is that fair? I have noted before that the absence of good product quality standards for dietary supplements is a persistent barrier to the use in science-based ways. Drugs have a rigorous standard that requires the products sold to be equivalent to the versions studied in clinical trials. With supplements, there is no similar quality standard. Weaker regulations and weaker standards mean less confidence for health professionals (and consumers) that what’s on the label is actually in the bottle.
The lack of confidence in quality seems justified. I recently described a disturbing study that suggested that in a sample of 44 supplements purchased off-the-shelf, only 48% were authentic and 59% of products contained species not listed on the label. Only two of the 12 companies in the sample had products without any substitution, contaminants and fillers.
The contamination concerns are among the most concerning – from lead in Ayurvedic remedies to shark cartilage supplements that contain mercury and neurotoxins and ironically, supplements contaminated with actual drugs. As long as there are poor quality standards, it’s difficult to see how supplements can be used safely in combination with prescription drugs, where an unlabelled ingredient could cause unanticipated harm.
The study
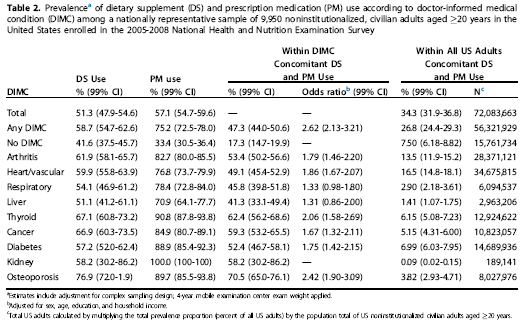
In light of the demonstrable quality problems with supplements, combining supplements and prescription drugs raises questions about safety. This new analysis, titled “Concomitant Dietary Supplement and Prescription Medication Use Is Prevalent among US Adults with Doctor-Informed Medical Conditions”, was published in the Journal of Academy of Nutrition and Dietetics in April 2014. The data comes from the NHANES database, which is supposed to be representative of your typical civilian, non-institutionalized adults. This survey excluded all pregnant women and participants were information was missing. The sample size was almost 10,000 adults.
NHANES assessed supplement use through personal interviews. Supplements were divided into 18 categories of products. Interestingly, multivitamins were divided into “standard” multivitamins (no minerals/botanicals) versus other forms of multivitamins, some of which also contained herbals. This makes sense, as there’s a big difference in ingredients between a cheap drugstore multivitamin and something like this which claims to be a detoxifying multivitamin. Prescription medication use was evaluated using the same process, as well as any “doctor informed medical conditions” (DIMC) which included:
- asthma
- arthritis
- congestive heart failure
- coronary heart disease
- angina
- angian pectoris
- heart attack
- stroke
- high blood pressure
- high cholesterol
- emphysema
- chronic bronchitis
- any liver condition
- thyroid problem
- cancer
- kidney dysfunction/dialysis
- osteoporosis
Both the term DIMC and the list of chronic illnesses included under this label seem to have been made up solely for this study – I could not find any other mention of it. Having said that, it is a reasonably good list of chronic medical conditions that require medical management and supervision.
The results
Those that used supplements and prescription drugs tended to be females >60 years old, college graduates, and hold higher income levels, which is consistent with what we’d expect with supplement users. Half of all adults used supplements, and more than half reported prescription drug use. And one third reported simultaneous supplement and prescription drug use. This would mean approximately 72 million Americans are taking prescription drugs and supplements together. The use of supplements or prescription drugs was higher among those with a DIMC – specifically osteoporosis, where almost everyone surveyed was taking supplements and prescription drugs:
Supplement use in oseteoporosis is not surprising, as the researchers categorized calcium as a “drug” if it was prescribed but as a “supplement” if it was purchased directly by the consumer without a prescription. A closer look at the supplements being used reveals some interesting data:
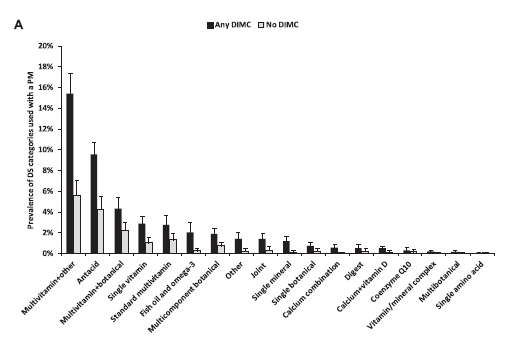
Notice how multivitamins and their variants make up the vast majority of supplement use. “Multivitamin plus other” (at least 3 vitamins and at least one other ingredient, but not a botanical) is the most prevalent, followed by antacids, and then multivitamin/botanical combinations. The use of products like single-ingredient botanicals was actually small – less than 1% of consumers. Looking at the relationship the other way, those taking cardiovascular drugs were the most likely to also be taking supplements, followed by those on central nervous system agents, then hormones, and so on:
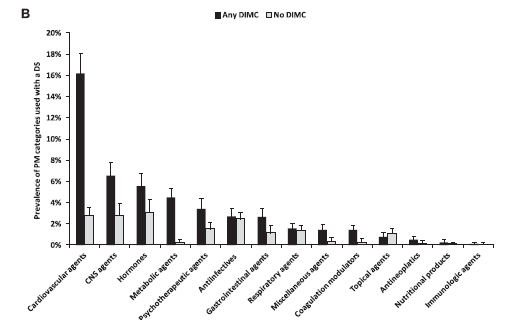
What this study tells me is that the most common supplement that’s combined with a prescription drug is usually a vitamin – but that vitamin may contain ingredients that go beyond the usual basic multi. (This isn’t the first survey that shows multivitamin use dominates supplement use.) The combination of botanicals into multi-vitamins is concerning, as it’s botanicals like herbs that can induce or impair liver enzymes and have dramatic effects on prescription drugs. Supplements like St. John’s Wort are the bane of pharmacists as they can cause an array of significant and severe drug interactions.
Conclusion
The concurrent use of supplements and prescription drugs is very common, even among those with major medical conditions. Yet there is limited data to suggest that supplements offer meaningful health benefits. Given the uncertain and unreliable quality of dietary supplements that are currently marketed, the risks and benefits of combining supplements with prescription drugs are not clear. Not only are there the potential harms from adverse effects from supplements, there is the additional risk of supplements interacting or interfering with the effectiveness of prescription drugs. Both consumers and health professionals need to understand that the decision to take supplements should not be made lightly – they may not be innocuous, and they may antagonize the effects of any concurrent prescription drugs. And even a multivitamin may contain ingredients that can antagonize medication. The exploding sales and use of supplements may be good business but the health effects are less clear. Mixing supplements and drugs is a type of health roulette – you simply can’t predict the effects.
Reference
Farina E.K., Austin K.G. & Lieberman H.R. (2014). Concomitant Dietary Supplement and Prescription Medication Use Is Prevalent among US Adults with Doctor-Informed Medical Conditions, Journal of the Academy of Nutrition and Dietetics, DOI: 10.1016/j.jand.2014.01.016
Photo from flickr user j.towbin © used under a CC licence.